Grups de recerca
Grups BBM

Grup d'Aplicacions Biomèdiques de la RMN (GABRMN)
Our major research interest is the improvement of the diagnosis, treatment and therapy response follow-up of abnormal brain masses, using noninvasive monitoring tools based in Nuclear Magnetic Resonance.
The large track of GABRMN in MR-based metabolomic studies, either in vivo, ex vivo or in vitro, is supported by solid results from group and its collaborators.Unravelling the potential of the metabolomics studies was always one of the main objectives of GABRMN. From the in vitro point of view, the group has worked with murine and human glioblastoma biopsies or cell lines, exploring high resolution approaches either through metabolite sample extraction or ex vivo analyses such as HRMAS (high resolution magic angle spinning) NMR.
Investigador principal: Ana Paula Candiota Silveira

Bases metabòliques del risc cardiovascular
El Grup de Bases Metabòliques del Risc Cardiovascular de l’Institut de Recerca del Hospital de la Santa Creu i Sant Pau, és un equip interdisciplinari i de recerca traslacional que treballa en el camp de les malalties cardiovasculars relacionades amb alteracions metabòliques como poden ser les dislipèmies, la diabetis mellitus, l’obesitat, les demències, o la hiperhomocisteinèmia, entre d’altres. La finalitat dels diferents estudis i línies d’investigació del grup es trobar noves teràpies dirigides i/o biomarcadors per tal de disminuir el risc cardiovascular i la malaltia d’Alzheimer en pacients amb aquestes alteracions metabòliques, així com millorar el diagnòstic i control dels pacients
Línies principals de Recerca i Innovació
- Disfuncionalitat del metabolisme de lípids i lipoproteïnes en la malaltia
- Nous biomarcadors i teràpies relacionades amb factors de risc cardiometabòlics, com ara l’obesitat, la diabetis, l’esteatohepatitis no alcohòlica i la hiperhomocisteïnèmia.
Investigador principal: Francisco Blanco Vaca

Biologia Estructural. Modificació de proteines
Our lab uses protein crystallography with synchrotron radiation as a major procedure to decipher the molecular mechanisms that lay behind the atomic structure of proteins and protein complexes. In our lab we combine this powerful structural technique with a functional and biochemical characterization using either in vitro or in vivo methods. In the last decades protein-function characterization of proteins and protein complexes have shed light into the most relevant discoveries in biochemistry and molecular biology.
Investigador principal: David Reverter Cendros
Biomarkers in Veterinary and Animal Science
The problems of the most important species from the productive, food and economic point of view (pig, cattle) from different approaches:
- Use of proteomic techniques to study these processes and identify biomarkers.
- Study of acute phase proteins: structure, function and utility as biomarkers.
- Role of brain neurotransmission in swine and cattle under stress and the influence of nutrition.
Investigadora Principal: Anna Maria Bassols Teixido
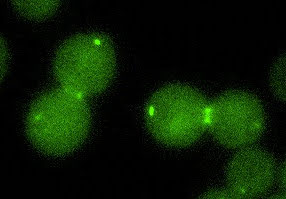
Cell cycle control pathways involved in the protection of genomic integrity, proliferation and cancer
Our interest is to identify novel cell cycle control elements and pathways involved in the protection of genomic integrity and the regulation of cell proliferation.
Investigador principal: David Garcia Quintana

Cell Death, Sencescence and Survival
|
Apoptosis is a complex but highly defined cellular program of cell demolition. Deregulation of apoptosis conducts to a number of serious diseases, including cancer. Biochemical events lead to characteristic cell changes and death. Activation of cysteine proteases called caspases plays a major role in the execution of apoptosis. These proteases selectively cleave vital cellular substrates, which results in the two hallmarks of apoptosis: nuclear chromatin condensation and internucleosomal DNA fragmentation. These hallmarks are achieved by a specifically activated DNase termed DFF40 or CAD (death fragmentation factor 40 or caspase-activated DNase). We are interested in the molecular mechanisms involved in the regulation of this endonuclease and the consequences for a cellular population derived from an abnormal or defective activation of DFF40/CAD. |
Investigador principal: Victor José Yuste Mateos

Cell Signaling and Apoptosis
Our work is mainly focused in the study of neurodegeneration in order better understand this extremely difficult process and help finding new treatments that can ameliorate the suffering of patients afflicted by these diseases, especially Alzheimer’s disease. One of our aims is to characterize the neurodegenerative process at the molecular level, in order to find new therapeutic targets to prevent neuronal cell death. In this regard, we have been focused in the last years in the study of the physiological and pathological roles of the anti-apoptotic neuronal protein and death receptor antagonist, FAIM-L. We have evidenced its interaction in neurons with XIAP and Siva-1, confirming that FAIM-L is involved in neuronal plasticity processes through stabilization of XIAP and modulating caspases activity. These results, added to our finding that FAIM-L controls neuroinflammation in Alzheimer’s disease (AD), allows positioning FAIM-L as an interesting target for the treatment of the disease. We have also demonstrated post-transcriptional regulation of faim gene by miRNAs altered in AD. In addition, we have completed the characterization of the in vivo faim knockout model, which has a surprising link with retinopathy. In particular, we have discovered that FAIM, with the two isoforms L and S, is abundantly expressed in retinal tissue, and that its absence in this tissue results in an elevated sensitivity to neurodegeneration, and in a dark adaptation delay, characteristic of several retinal pathologies. We are further characterizing the interaction of FAIM-L with other relevant proteins for neurodegeneration, and its role in human diabetic retinopathy. We are also developing tools to characterize FAIM-L expression and modulating its levels in different animal models of AD.
Investigadora Principal: Montserrat Solé Piñol

Epigenetic regulation of chromatin structure
Our research is focused on histone H1 and its role in the epigenetic regulation of chromatin higher-order structure and dynamics. In humans, there are seven H1 somatic subtypes or variants. H1 subtypes are present in different proportions, depending on the cell type and stage of development. While H1 subtypes can compensate for the H1 depletion of one or two subtypes, numerous studies suggest that they are functionally distinct. H1 subtype composition is also altered in pathological states, especially in cancer, highlighting the importance of H1 for cellular homeostasis. Therefore, we are interested in studying the regulation of the expression of H1 subtypes in health and disease. Our findings may provide further evidence of the functional differentiation of H1 subtypes, the compensatory effects upon H1 perturbation, and the role of H1 in carcinogenesis.
Investigadora Principal: Alicia Roque Cordova

Gene Therapy for Nervous System (GT4CNS)
|
La Medicina encara té molts reptes per resoldre, especialment en malalties complexes a on hi ha un gran nombre de factors genètics i ambientals implicats. Entre ells, els trastorns neurodegeneratius i autoimmunes que afecten el sistema nerviós central han atret l'atenció perquè el SNC és difícil d'accedir i manipular amb els tractaments farmacològics clàssics i, a més, no hi ha tractaments curatius eficaços per a aquestes malalties. Per fer front a aquests problemes hem centrat els nostres interessos en estratègies de teràpia gènica per a malalties autoimmunes i trastorns neurodegeneratius, especialment aquells associades amb l'envelliment. Finançament: ISC-III (# PI15-01270); per COMRDI15-1-0013-16 (Amb el suport d’ACCIÓ en el marc del Programa Operatiu FEDER Catalunya 2014-2020), la Fundació Marato TV3, Convenis amb empreses privades. Molecular mechanisms involved in memory formation: Study of altered signaling pathways in CNS in aging and in Alzheimer's Disease Gene Therapy Strategies for Neurodegenerative rare diseases: Spastic Paraplegia type 52 (SPG52); and GNB1 Encephalopathy Generation and development of new AAV gene therapy vectors |
Investigador principal: Miguel Chillón Rodríguez

Gene Therapy for Neurometabolic Diseases
The research activity of our group is focused on the development of gene therapy strategies for rare diseases affecting the nervous system, both central (lysosomal storage diseases, ALS, Wolfram Syndrome and MLC) and peripheral (genetic and acquired neuropathies) and on the elucidation of the molecular mechanisms implicated in the development of these pathologies combining the use of animal models, tissue cell culture and viral vectors.
Investigadora Principal: Assumpció Bosch Merino

Human Host Defence RNases
Our lab is working on the development of novel antibiotics based on the structure-functional knowledge of human secretory RNases.
Human secretory RNases are key players of the host immunity and contribute to maintain the body fluids’ sterility. They are activated upon a diversity of cellular stress injuries and mediate signaling processes, classified therafter as alarmins. Interestingly, secreted RNases can shape the non-coding RNA population and participate thereby in the host innate immune response. We are currently exploring both the immuno-modulation and anti-infective activities of human canonical RNases. Structural- functional analysis is applied in the design and engineering of new scaffolds to develop novel antibacterial and antiviral agents. In particular, we are aiming to target microbial resistance forms, such as biofilm communities and macrophage dwelling pathogens.
Investigadora Principal: Ester Boix Borras
Interactomics in Physiopathology: Proteins, Peptides and Membranes
In humans, cells and cellular compartments use membrane proteins (MP) as the first sensors and actuators towards the environment. Cells communicate with other cells, pathogens, or the media by means of membrane proteins. Thus, it is key to know how membrane proteins exert their function, especially considering that most of the pharma industry therapeutic targets are membrane proteins, such as ion channels, GPCRs, transporters, etc. Structure and function of membrane proteins are intrinsically related, and membrane protein structural biology is a challenge, thus out-of-the-box strategies are required to understand the interactions of proteins and membranes and the implications in pathophysiology. In order to gain this knowledge, we propose a multidisciplinary and multiperspective approach combining wet and dry lab experiments.
Investigador Principal: Alex Peralvarez Marin

Laboratori d'Enginyeria Genètica Animal
Our main strategic objective is to develop new gene therapy approaches for highly prevalent and rare metabolic and neurodegenerative diseases, with the ultimate goal to improve the quality of life for subjects affected by these diseases. Our studies focus on the pathophysiological causes of diabetes mellitus and its comorbidities and also of severe genetic storage diseases, generating transgenic animal models and developing gene transfer-based therapeutics.
Investigadora Principal: Maria Fatima Bosch Tubert

Lesch Nyhan Disease
We are interested in how cell metabolism, signalling pathways, and gene expression are rewired in pathological conditions such as Lesch-Nyhan disease, a metabolic illness with severe neurological manifestations, or in cells submitted to hyperosmotic shock, a common stress that cells have faced during millions of years since their emergence.
We study the cellular abnormalities in Lesch-Nyhan disease and the potential treatments to revert these alterations. We also aim to understand the mechanisms that regulate osmostress-induced apoptosis and meiotic progression, and the role of stress protein kinases in these processes.
Investigador Principal: Jose Manuel Lopez Blanco

Lipid-based nanosized drug delivery systems
Phospholipid aggregates are in common use as universal drug delivery carriers. Modulation of their physicochemical properties enables the design of particular systems with desired properties.
Currently we are working on the preparation and characterization of mixed systems constituted by phospholipids and molybdenum carbonylic metallosurfactants, a particular molecule which exhibits CO releasing properties. In recent years it has been corroborated that, at certain levels, local delivery of CO exhibits therapeutic effects, for example, as anti-inflammatory, in cardiovascular diseases and also in organ transplantation
Investigador Principal: Ramon Barnadas Rodriguez

Metabolism and calcium signalling in brain
Our group is interested in signalling and metabolism in the brain at multiple levels, from single astrocytes, astrocyte-neuron interactions and neuronal circuits, to control of behaviour and memory. We are also interested in how these processes are altered in neurodegeneration, in particular Alzheimer’s disease, to elucidate multi-targeted and multi-cellular therapies.
Investigadora Principal: Roser Masgrau Juanola

Metabolomics and Imaging Data lab (MIDAlab)
Using metabolomics combined with imaging data to discover diagnostic and prognostic biomarkers for brain diseases.
We focus on magnetic resonance (MR) data analysis, and particularly in magnetic resonance spectroscopy (MRS), alone or in combination with other MR-related imaging modalities, using clinical data from collaborating hospitals and clinical centres or preclinical data.
Investigadora Principal: Margarita Julia Sape

Molecular Biology of Synaptic Dysfunction in Neurodegenerative Diseases
The basic objective in our lab has been to study the molecular and cellular mechanism involved in neuronal death, as a direct approach for the understanding of neurodegenerative diseases. Although neuronal death in neurodegenerative diseases is very complex, it seems that certain characteristics are common between them. For example, it seems quite clear that apoptosis contributes to cell death observed in Alzheimer, Parkinson or Hungtinton diseases. We are focused in the study of regulation of neuronal apoptosis. Two questions are being addressed in the lab? 1.- Which is the role of apoptosis in cerebral ischemia-mediated cell death 2.- How synaptic activity and some extracellular factors are able to reduce neuronal apoptosis in neurodegenerative diseases?
Investigadors Principals:


Neurobiology of Alzheimer's disease
Alzheimer´s disease (AD) is characterized by accumulation of phosphorylated tau and β-amyloid (Aβ) peptides in brain regions encoding memory. Aβ is generated by presenilin/γ-secretase-mediated cleavage of the β-amyloid precursor protein. Our investigations focus on molecular mechanisms regulated by presenilins and Aβ that alter gene expression programs leading to synaptic dysfunction and memory loss in Alzheimer´s disease.
Investigador principal: Carlos Saura

Oxidoreductases in Cellular Defense and Signaling. Search of inhibitors for therapeutic purposes
Our current research is focused on the enzymology and molecular biology of oxidoreductases involved in mechanisms of cellular defense and signaling. We are investigating members of three oxidoreductase superfamilies participating in the metabolism of carbonyl compounds, along with other biological functions: aldo-keto reductases (AKR), medium-chain dehydrogenases/reductases (MDR) and aldehyde dehydrogenases (ALDH). We are especially interested in enzymes acting on the metabolism of signaling molecules (retinoids and prostaglandins) and associated with different human disease states, such as cancer and diabetes. Some of these enzymes are also phase I-drug metabolizing enzymes and are induced under oxidative stress, being responsible for developing tumor resistance to chemotherapy. Thus, they are relevant drug targets and are well suited for the design of selective enzyme inhibitors with potential use as pharmacological agents. Some illustrative examples of the enzymes being studied are zeta-crystallin, an RNA-binding protein involved in the cellular stress response; pro-apoptotic p53-induced gene protein 3 (PIG3); and aldo-keto reductase 1B10 (AKR1B10), a highly efficient retinaldehyde dehydrogenase which is induced in several types of cancer.
Investigador principal: Jaume Farrés Vicen

Plant Environmental Epigenetics
Environmental epigenetics aims to understand how abiotic and biotic factors affect the regulation of development without directly changing the genetic code. The mechanisms underlying this regulationare chromatin-based and most of them rely on epigenetic marks, suchhistone modificationsor DNA methylation.In the Plant Environmental Epigenetics lab we studyhow epigenetics changes modulate plant growth and stress tolerance in response to environmental stimuli. Therefore, the experimental work developed is onthe crosstalk between plant signalling responses and chromatin status
Investigador principal: Jordi Moreno Romero

Protein Engineering and Nanomedicine Lab
Our research group focuses on protein engineering towards the generation of functional nanocarriers and bioinspired nanomaterials with potential applications in both nanomedicine and nanotechnology. We are particularly interested in the development of biocompatible nanomaterials and the study of their biological properties and interactions under clinically relevant conditions. Our primary goal is to address the challenge of delivering these biocompatible nanocarriers to the brain via intranasal administration, which would enable effective treatment of neurological disorders such as glioblastoma and Parkinson's disease.
Investigador Principal: Julia Lorenzo

Protein Folding and Conformational Diseases
Our laboratory employs a multidisciplinary and collaborative approach to studying protein folding, misfolding, and aggregation. Our research aims to uncover the underlying mechanisms of these processes and their association with human conformational diseases, such as Parkinson’s disease, and orphan diseases related to amyloid deposition. Our primary objective is to develop novel therapeutics and diagnostic tools to target these pathologies effectively.
Furthermore, our expertise in protein folding and aggregation enables us to design and manufacture advanced protein-based biopharmaceuticals. Additionally, we are dedicated to exploring the potential of self-assembled functional materials for nanotechnology applications, including biocatalysts, biosensors, drug delivery, and immunotherapy.
Investigador Principal: Salvador Ventura

Protein kinases in Cancer Research
Lizcano’s Lab is interested in dissecting new cellular signaling pathways that control cancer cell proliferation and differentiation. We collaborate with academics and Biopharma Companies to perform preclinical development of new anticancer drugs. Specifically, we are interested in
deciphering the role of the new MAP kinase ERK5 in cancer proliferation and survival. We are also interested in modulation of autophagy and endoplasmic reticulum (ER) stress as new strategies to tackle cancer.
Investigador Principal: Jose Miguel Lizcano

Self-organization in biological systems
Our lab is focused on understanding the self-organization of protein mixtures through the study of their interactions at the molecular level. In this sense, protein interactions can result in differently organized assemblages with diverse biophysical properties and biological purposes. They can build strong and resistant amyloid fibrils with structural, adaptative and epigenetic purposes. But also, active, sensitive and modulable biological condensates with metabolic and regulatory functions.
Investigadora Principal: Natalia Sánchez

Signalling in the central nervous system
Neurons born from progenitor cells progress to their mature phenotype by means of intricate genetic programs which controls cell survival, growth, differentiation, migration and synapses formation. During this process, neurons encounter a great diversity of growth factors and hormones that activate different signal transduction pathways that ultimately control the physiology of the cell. Among them, the PI3K-PKB pathway emerged during the last decade as a central mediator of neurotrophic factor-induced neuronal survival, and PKB is considered nowadays as a drug target to treat neurodegenerative disorders. However, most of the studies are based in the use of specific inhibitors of the phosphoinositide 3-kinase (PI3K) combined with overexpression experiments of dominant negative and constitutively active forms of the protein kinase B (PKB). Since specific PKB inhibitors are not available at present, genetic evidences demonstrating a role of PKB in regulating neuronal survival are needed.
Investigador Principal: Jose Ramón Bayascas

Structural Biology of Alzheimer's Disease. Biophysical studies
My research’ group activity is dedicated to the characterization of amyloid peptides and proteins related to conformational diseases and to the study of dendrimers as antiamyloidogenic agents. The studies on amyloid peptides related to conformational diseases have been carried out in close collaboration with Núria Benseny and three other important collaborations: (1) Prof. I. Ferrer’s laboratory at IDIBELL (Barcelona); (2) Profs. M. Bryszewska and B. Klajnert, at the University of Lodz (Poland); (3) Dr. Dietmar Appelhans, at the Institute of Polymer Research in Dresden (Germany).
Investigador Principal: Jose Bartomeu Cladera

Synaptic pharmacology lab
Basic knowledge of the biochemical targets for psychotropic drugs. This includes dopaminergic and other neurotransmitter receptors, transporters and enzymes affected by antipsychotics, drugs of abuse and treatments for dyskinesias.
Investigador Principal: Jordi Ortiz

Systems Biology of Infection Lab
We are interested in understanding bacterial pathogenesis at the system architecture level. We aim to identify and characterise host-pathogen protein-protein complexes, understand why these complexes are relevant for the disease and how they are dynamically regulated during infection. We are addressing these challenges by using a combination of high-throughput methods together with mathematical and modelling approaches. We aim to use these knowledge to design new antibiotics, particularly against multi-drug resistant bacteria.
Investigador principal: Torrent, Marc

Yeast Molecular Biology Laboratory
Our group is interested in several topics about the biochemistry, the molecular biology and the genomics of the yeast Saccharomyces cerevisiae, specifically those that are related to cell signalling trough processes of phospho-dephosphorylation. For this purpose, we investigate issues like the ion homeostasis, the response to various stresses or the cell cycle regulation. The main objective is to obtain an overview on the yeast response to perturbations of its environment, so that we can both understand in deep the biology of this organism and to guide us towards new biotechnological applications.
Investigador principal: Ariño, Joaquin
Pàgina web