New article: "Meiotic cohesin RAD21L shapes 3D genome structure and transcription in the male germline"
Research Group: Genome Integrity and Instability
Abstract:
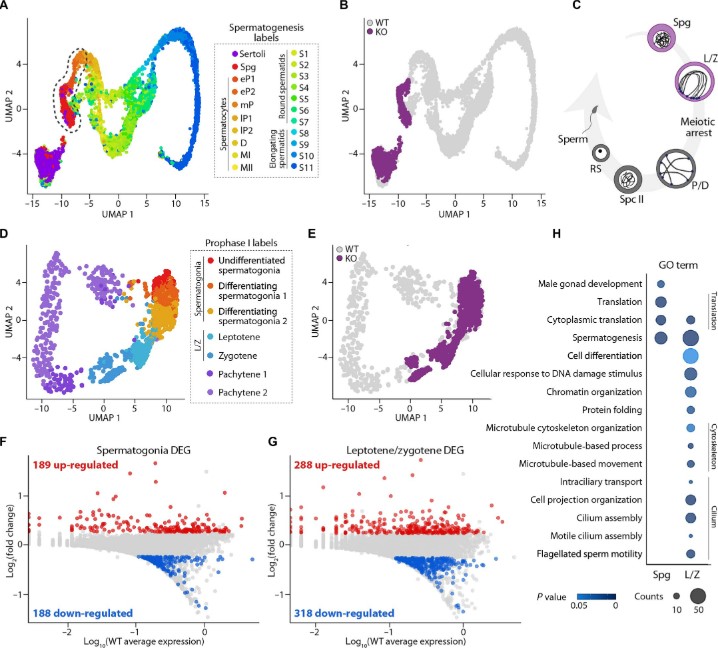
The distinctive three-dimensional (3D) chromatin architecture adopted by chromosomes during meiosis is essential for fertility, yet the functional implications of the fine-scale spatial organization remain poorly understood. Here, we investigate the impact of RAD21L deletion, a meiosis-specific cohesin subunit, on the 3D genome architecture and gene expression in the male germ line. Using fluorescence-activated cell sorting, high-throughput chromosome conformation capture, and single-cell RNA sequencing, we demonstrate that RAD21L deficiency impairs meiotic chromatin organization and inter/intrachromosomal interactions. This conspicuous 3D genome reorganization also disrupts bouquet formation, resulting in increased telomeric interactions between heterologous chromosomes in primary spermatocytes. Genome reorganization was accompanied by detectable transcriptional dysregulation in spermatogonia and primary spermatocytes, mainly affecting sex chromosomes. Collectively, our findings establish the cohesin RAD21L as a germline-specific critical regulator of genome-wide 3D genome reorganization during spermatogenesis and a modulator of the male germline transcriptional landscape.
Full news access:
https://www.uab.cat/web/newsroom/news-detail/discovery-of-how-a-protein-regulates-dna-and-affects-male-fertility-1345830290613.html?detid=1345967406528
Article data:
Laia Marín-Gual et al. Meiotic cohesin RAD21L shapes 3D genome structure and transcription in the male germline.Sci. Adv.11,eadv2283(2025).
The UAB, with the Sustainable Development Goals
-
Good health and well-being