New pyrazole-ether hybrid ligands in palladium catalysts
Catalysts are important as they increase the speed of chemical reactions. If these compounds are coordination complexes, their structure and ligands present in the composition are fundamental when defining their behaviour. The research group led by Fina Pons from the Department of Chemistry has synthetised new pyrazole-ether N1-substituted hybrid ligands for palladium catalysts involved in the Heck reaction.
The development of science and technology in recent decades has been definitely related to the oil industry, due to the large amount of materials and products obtained from it. However, this development would be practically impossible, without the catalysis. Nowadays, 90% of chemical transformation processes of oil are catalytic [1].
Catalysis is a phenomenon which involved some substances, called catalysts, in chemical reactions where, without their presence, these reactions occurred in a very slow way [2]. The Heck reaction is one of the most widely used reactions for the formation of carbon-carbon bonds in organic synthesis using, as a catalyst, a complex of palladium [3].
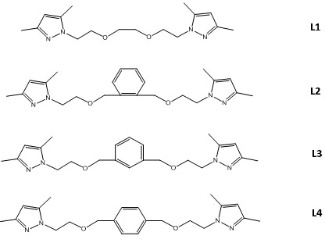
Previously, our research group has studied different complexes of Pd (II) with ligands derived from pyrazole and they have shown to act as efficient catalysts for the Heck reaction [3]. As an extension of this research, we have tested the catalytic properties of different compounds in the cross-coupling reaction between aryl halides and different alkenes, to study the effect caused by the different structures of the new ligands N1-substituted (L1-L4) in the activity of the catalyst (see figure on the left). It has been shown that the best catalyst is the one formed by the ligand L1, active even for aryl chlorides. These results are consistent with those obtained previously, where it was reported that this ligand has more flexibility and versatility than its analogs, because it is able to adapt to a wide range of coordination geometries (tetrahedral or cis-trans square planar, octahedral) and coordination modes like N, N-bidentate (chelated or bridging) or N, O, O, N-tetradentated (equatorial or axial) [5].
To sum, this family of compounds has many advantages since it does not contain phosphine ligands groups in their structure, they are stable to air and temperature, and therefore can form a practical and efficient catalytic system under mild reaction conditions.
[1] G. C. Laredo, J. O. Marroquin, J. Castillo, P. Perez-Romo, J. Navarrete-Bolaños, Appl. Catal. A. Gen., 2009, 363, 19.
[2] S. T. Oyama, T. Gott, H. Zhao , Y.-K. Lee, Catal. Today, 2009, 143, 94.
[3] R. F. Heck. Palladium Reagents in Organic Synthesis, Academic Press: London, UK. 1985
[4] V. Montoya, J. Pons, J. García-Antón, X. Solans, M. Font-Bardía. J. Ros, Organometallics, 2007, 26, 3183.
[5] M. Guerrero, J. Pons, T. Parella, M. Font-Bardia, T. Calvet, J. Ros. Inorg. Chem. 2009, 48, 8736.
References
"Effect of N1-substituted pyrazolic hybrid ligands on palladium catalysts for the Heck reaction". Guerrero, Miguel; Pons, Josefina; Ros, Josep. JOURNAL OF ORGANOMETALLIC CHEMISTRY, 695 (17): 1957-1960 AUG 1 2010.

