New molecules with ionic liquid crystal properties
Ionic liquid crystals can be regarded as materials that combine the properties of liquid crystals and ionic liquids.
On one hand, ionic liquids are compounds composed of ions, which have relatively low melting points. They are characterized by extremely low vapor pressure, wide liquid ranges, nonflammability, thermal stability, tunable polarity and easy recycling. Recently, ionic liquids have attracted great interest as environmentally friendly solvents to replace common organic solvents because of their low volatility. One of the most used cations is imidazolium. We have synthesized salts with two or three imidazolium moieties in their structure (Figure 1). Some of their properties such as solubility or melting point have been modulated by changing the anion.
On the other hand, liquid crystals are compounds that present an intermediate phase between the solid one and the liquid. In a general way, three states of matter are considered, solid, liquid and vapour. When an organic compound is in its solid state, the most ordered state of matter, molecules are in a fixed position but when the liquid state is reached the positional order is broken and the molecules can move freely. Certain substances melt giving another state of matter called liquid crystal phase or mesophase. This mesophase has properties and characteristics of the solid state and of the liquid state. In a liquid crystal the molecules are oriented around a preferred direction. One of the main applications of liquid crystals is the manufacture of displays (LCD, Liquid Crystal Display).
Most of the molecules synthesized in this work (Figure 1) present a smectic A phase. When X is I or BF4 the molecules present a wide range of liquid crystal. For instance, molecule 1 when X is BF4 is a white solid at room temperature, liquid crystal between 43 �C and 150 �C and then melt as a liquid.
Figure 1. Molecules synthesized in the present work.
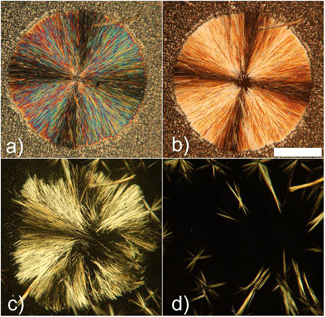
Figure 2. Polarized optical micrograph of the liquid crystal phase (smectic A phase) of compound 1 when X is BF4 at 140 �C.
References
Ionic liquid crystals based on mesitylene-containing bis- and trisimidazolium salts. Montserrat Trilla, Roser Pleixats, Teodor Parella, Christophe Blanc, Philippe Dieudonné, Yannick Guari, Michel Wong Chi Man. LANGMUIR, 24 (1): 259-265 JAN 1 2008.

