New molecular mechanism in DNA repair
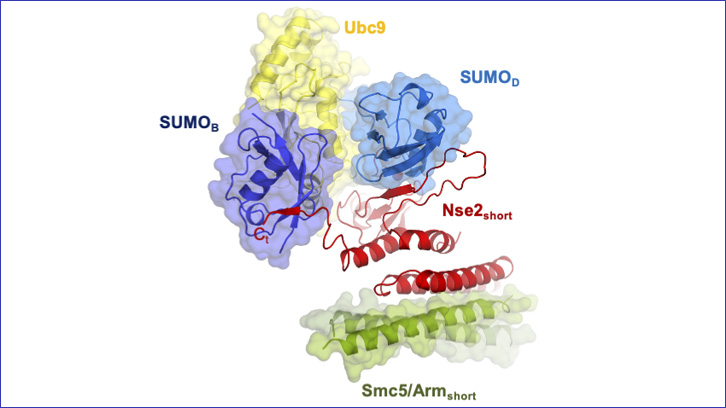
A study led by a researcher teams from the IBB and the Department of Biochemistry and Molecular Biology of the UAB has revealed the functioning of an enzyme complex formed by a set of proetines -an E3 ligase, linked to an E2 and two SUMOs, identifying the key points for the correct function of this E3 ligase that participates in the repair of DNA damage through post-translational modifications by SUMO.
Post-translational modifications of proteins are those changes that are made once the protein has already been synthesized by the ribosome. These modifications are very important, since they can produce changes in the biological activity of the modified proteins, thus generating an important cellular regulation mechanism.
SUMO (Small Ubiquitin-like MOdifier) is a small protein that has the ability to bind to other proteins through these post-translational modifications, giving rise to a process called “SUMOylation”. There are a large number of proteins involved in SUMOylation, necessary for the activation of SUMO itself and also subject to this post-translational modification. Among them, the Smc5/6 complex stands out, a complex formed by various proteins that actively participates in the repair of DNA damage.
In this article, we present the three-dimensional structure of two proteins of the Smc5/6 complex: Nse2 and Smc5, interacting with two key proteins in the SUMOylation process: E2 and the SUMO protein itself, linked by a thioester bond. Thanks to the observation of this enzymatic complex, we have been able to define the key points of the union between these proteins that have allowed us to characterize the mechanism of the SUMO E3 ligase activity of Nse2 and its implication in DNA repair.
This work has been carried out by the research group led by Dr. David Reverter, linked to the Institute of Biotechnology and Biomedicine and the Department of Biochemistry and Molecular Biology of the UAB, in collaboration with the group of Dr. Jordi Torres-Rosell, linked to IRB-Lleida. The first authors of the article are Dr. Nathalia Varejão and the predoctoral researcher Jara Lascorz, also belonging to the IBB-UAB.
Institut de Biotecnologia i de Biomedicina
Department of Biochemistry and Molecular Biology
Universitat Autònoma de Barcelona
References
Varejão, N., Lascorz, J., Codina-Fabra, J., Bellí, G., Borràs-Gas, H., Torres-Rosell, J., & Reverter, D. (2021). Structural basis for the E3 ligase activity enhancement of yeast Nse2 by SUMO-interacting motifs. Nature Communications, 12(1), 7013. https://doi.org/10.1038/s41467-021-27301-9


