Manganese complexes to improve magnetic ressonance images
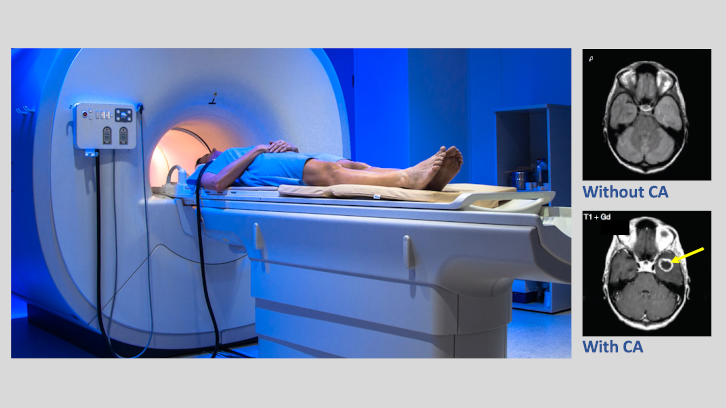
Many times the contrast level of Magnetic resonance images (MRI) is not enough for unequivocal diagnosis and, for this reason, the so-called contrast agents (CAs) are used. Rosa Ortuño, researcher at the Department of Chemistry, explains how the synthesis of manganese complexes provide a contribution to the search of effective CAs for clinical purposes.
Magnetic resonance imaging (MRI) is a powerful tool for clinic diagnostics with a great relevance in medicine, thus justifying the development of an active research in this field carried out by multidisciplinary teams. This technique is possible because of the magnetic properties of the protons present in water molecules, which is plentiful in the human body. Nevertheless, many times the contrast level of images is not enough for unequivocal diagnosis and, for this reason, the so-called contrast agents (CAs) are used. They are based on paramagnetic metal complexes, such as gadolinium, manganese, iron, and others. Once they are injected into the human body, the metal can coordinate with water molecules to fulfill its coordination sphere, contributing in this way to increase the sensibility and the clarity where there is a larger aqueous accumulation as, for instance, in a tumor. The result is like a black and white photograph with enhanced contrast.
Good CAs must present some requirements such their kinetic stability (inertness), thermodynamic stability, resistance to the interchange with endogenic ions present in biological medium, such zinc or coper cations and phosphate and carbonate anions. On the other hand, the hydration number (number of water molecules, 1-3 in the most common cases) play a relevant role, as well as the water exchange rate. Faster exchange improves the AC efficiency.
The first CAs were based on gadolinium complexes with macrocyclic ligands since rigidity favors the aforementioned properties, mainly stability. The most widely used is Dotarem® that is a commercial product.
Our research group in UAB, in collaboration with experts from Universidade da Coruña and the Centre of Biophysique Moléculaire-CNRS in Orléans, prepared and investigated in an earlier work [1] a family of gadolinium complexes including acyclic ligands that contain carbon-four-membered rings (cyclobutanes), which confer with a high rigidity to these compounds. The results from this study, by means of spectroscopy, spectrophotometry and potentiometry, jointly with computational calculations, corroborated that their stability and efficiency are equal or higher than those of some CAs already approved for clinical uses.
However, FDA (Food and Drug Administration) in USA and agencies in other worldwide countries have recently restricted the clinical use of gadolinium complexes because of their high toxicity and in vivo potential metal release. Indeed, nephrogenic systemic fibrosis is a pathology directly linked to gadolinium injections and gadolinium accumulation in brain and bones of patients with frequent exposure to contrast-enhanced MRI have been found. Hence, these facts reinforced the exploration of new efficient and stable CAs based on other more biocompatible elements.
In the more recent work [2] we described the synthesis of manganese complexes with the same ligands than those reported in the previous work. Although these new complexes showed a lower stability than those with gadolinium, the results concerning their efficiency are very good and provide a contribution to the search of effective CAs for clinical purposes.
References
[1] Oriol Pocar-Tost,a José A. Olivares,a Agnès Pallier,b David Esteban-Gómez,c Ona Illa,a Carlos Platas-Iglesias,c Éva Tóth,b Rosa M. Ortuño.a Gadolinium complexes of highly regid, open-chain ligands containing a cyclobutane ring in the backbone: Decreasing ligand denticity might enhance kinetic inertness. Inorg. Chem. 2019, 58, 13170-13183. DOI: 10.1021/acs.inorgchem.9b02044.
[2] Oriol Pocar-Tost,a Agnès Pallier,b David Esteban-Gómez,c Ona Illa,a Carlos Platas-Iglesias,c Éva Tóth,b Rosa M. Ortuño.a Stability, relaxometric and computational studies on Mn2+ complexes with ligands containing a cyclobutane scaffold. Dalton Trans. 2021, 50, 1076-1085. DOI: 10.1039/d0dt03402a.
a Departament de Química, Universitat Autònoma de Barcelona, 08193 Cerdanyola del Vallès, Barcelona, Spain. E-mail: rosa.ortuno@uab.cat
b Centre de Biophysique Moléculaire, UPR 4301, CNRS, Université d’Orléans, rue Charles Sadron, 45071 Orléans Cedex 2, France. E-mail: eva.jakabtoth@cnrs.fr
c Universidade da Coruña, Centro de Investigacións Científicas Avanzadas (CICA) and Departamento de Química, Facultade de Ciencias, 15071 A Coruña, Galicia, Spain. E-mail: carlos.platas.iglesias@udc.es

