The fuel that makes water, not fire
The hydrogen cell can be the source of clean energy that replaces fossil fuels, but the component where the chemical reactions take place, the catalyst, requires the use of platinum, a very expensive material. Researchers from the Department of Physics, in collaboration with a research team from Sweden, have built a cheaper catalyst that uses platinum more effectively and have successfully tested it in a prototype fuel cell.
We did some research on catalyst materials for a fuel cell. But what is a fuel cell actually? The term is quite misleading, and might suggest that it needs common fuel, like gasoline*. But far from it! The only actual fuel here is hydrogen gas, which does not consume any resources at all. And the best of it, the fuel cell runs completely emission-free. Inside the cell, the chemical energy stored in hydrogen is converted into electric power, while hydrogen and oxygen combine to water. And from the water, we can produce green hydrogen gas again. In this way, hydrogen has an endless life cycle as an energy carrier, and the fuel cells can be used to power just about anything, from smartphones up to heavy duty trucks.
In our research group, the Group of smart nanoengineered materials, nanomechanics and nanomagnetism (Gnm3) at the UAB, we are constantly thinking about how to improve green hydrogen energy, such as fuel cell technology, and test our ideas experimentally. While we work a lot on the synthesis of catalysts for green hydrogen production, our colleagues at the Research Institutes of Sweden (RISE) are specialised on fuel cells, and build the bridge from R&D to industry. Therefore, we decided to take advantage of both parties’ expertise to bring our laboratory-scale materials to an actual application in a fuel cell prototype.
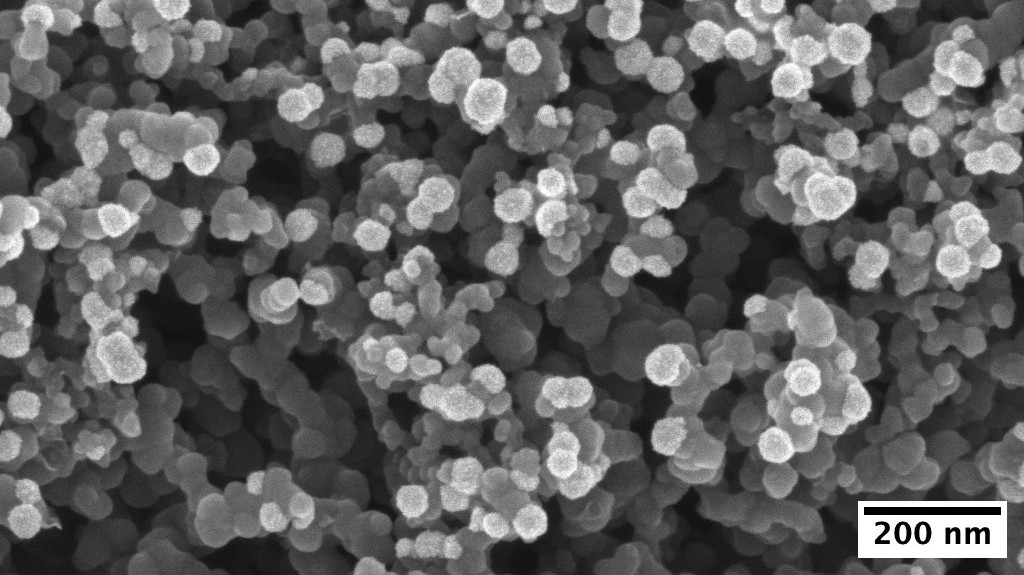
We produced catalysts made of platinum-nickel nanoparticles, some of them containing molybdenum. The catalyst is the most important ingredient of the fuel cell. It is exactly where the chemical reactions take place. In operation, the catalyst is neither consumed nor altered in any way – in theory. The truth is that fuel cell catalysts suffer from various forms of degradation. While tackling this we also want to make the catalysts more efficient and less expensive. Fuel cells work in harsh environments, which is why there is hardly any way around the use of expensive platinum, but there is certainly potential to reduce the cost of fuel cells by making the platinum in them work more efficiently. This is why we alloy platinum with nickel and molybdenum, for one part. On top of that, with the electrochemical synthesis method we employed, we manage to place the nanoparticles at the locations where they are most effective. Our method even has a pleasant side effect: the catalyst is synthesised at the same time as it is being distributed onto the part of the fuel cell it belongs to, and the fuel cell can be assembled in a few simple steps. In conventional fuel cells, many catalyst nanoparticles are actually not available for the chemical reactions because they are simply not in the right place.
As a result, the efficiency of our catalysts with respect to the used amount of platinum is extremely high. Together with RISE's researchers, we could successfully prove the functioning of our catalysts in a fuel cell prototype. Although much research is still to be done, our work can help to make fuel cells more affordable, and thus to build an infrastructure for green hydrogen that can eventually replace fossil fuels completely.
*Definition of fuel in the Oxford dictionary: “Material such as coal, gas, or oil that is burnt to produce heat or power.”
Konrad Eiler1, Jordi Sort1,2 i Eva Pellicer1
1Department of Physics, Universitat Autònoma de Barcelona
2Institució Catalana de Recerca i Estudis Avançats (ICREA)
References
K. Eiler, L. Mølmen, L. Fast, P. Leisner, J. Sort, E. Pellicer, Oxygen reduction reaction and PEM fuel cell performance of pulse electrodeposited Pt–Ni and Pt–Ni–Mo (O) nanoparticles, Materials Today Energy, 2022, 27, 101023; 10.1016/j.mtener.2022.101023