Synthesis of coordination polymers and zinc compounds
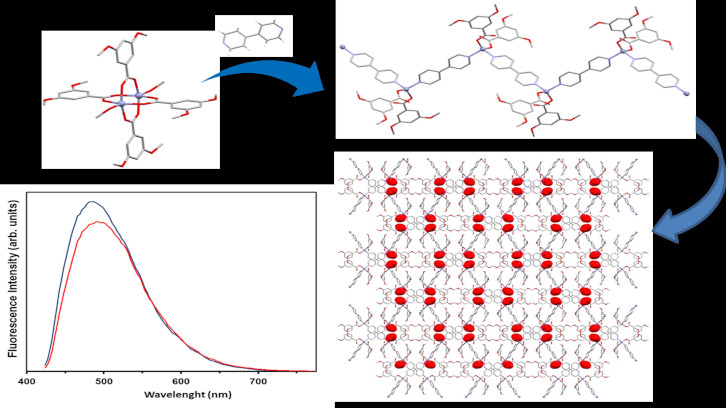
4,4'-bpy is one of the ligands that, together with the carboxylate type, allows to obtain coordination polymers. The present work focuses on the influence of these two ligands to determine the structure of zinc compounds and designed polymers. The complexity of the final structure of these, however, complicates obtaining them and requires considering various parameters and carrying out many reactions. In addition, the luminescence which present these polymers (blue/green). They can be used for photoactive materials.
Coordination polymers (CPs) have attracted an enormous interest due to their interesting physical and chemical properties. The 4,4’-bipyridine (4,4’-bpy) is an exo-bidentate ligand which act as an ideal linker between transition metals in order to obtain coordination polymers. The structure of the polymers depends on the geometric preference of the metal ions and on the metal-to-ligand molar ratio. It can promote the formation of a great variety of frameworks, varying from 1D to 3D nets.
When the proportion is 1:1 metal-to-ligand molar ratio usually generates linear zigzag chains, while the 1:2 metal-to-ligand molar ratio tends to form square grid or diamond-like structures, among others. Besides, in the obtention of different structures, it has been observed the influence of the other factors as anionic ligands, pH value and solvent used in the synthesis and recrystallization of the compounds. In some cases, a subtle alteration in any of these factors can lead to complexes with different structure and/or dimensionality.
The 4,4’-bpy ligand acts as bridging but can also act as a terminal ligand or as an uncoordinated guest molecule, which may be further involved in hydrogen bonding and/or π-π stacking interaction.
The supramolecular structures made from the aggregation of 1D polymers can exhibit cavities/channels of molecular dimensions and thus, include different guest molecules. All these possibilities explain the difficulty to define the suitable experimental conditions for the formation of a coordination polymer with a desired structure, because there are many synthetic parameters needed to be considered.
The carboxylate ligands constitute and important class of ligands in the formation of CPs and concretely one of the used in the construction of CPs in combination with 4,4’-bpy. The presence of the OH- groups in carboxylate ligands determine the arrangement of the polymer chains, as hydroxyl groups can be involved in the formation of hydrogen bond intermolecular interactions.
The finality of this paper is to study the influence of different carboxylate ligands with 4,4’-bpy in the structure of the zinc complexes. The compounds [Zn(3,5-(MeO)2Bz)2(CH3OH)]2 (3,5-(MeO)2Bz = 3,5- dimetoxibenzoat) and [Zn(µ-3,5-(OH)2Bz)(µ-OH2)(H2O)2] (3,5-(OH)2Bz = 3,5-dihydroxybenzoate) were used as precursors for the synthesis of CPs. The four compounds were synthesized, and it has been demonstrated the influence of the solvent in the obtention of one and another CPs.
All obtained compounds were characterized by analytical and spectroscopic techniques. For all compounds, their crystal structures were elucidated reveling 1D coordination polymers in zigzag arrays. Also were analyzed the extended structures and recorded and analyzed the luminescence spectra in solid state, obtaining a strong luminescence (blue/green). They may be as potential photoactive materials.
Universitat Autònoma de Barcelona.
Chemistry Department.
Inorganic Chemistry Area.
References
Francisco Sánchez-Férez, Roger Pou,Laura Bayés-García, Mercè Font-Bardía, Josefina Pons, José A. Ayllón. Benzoate substituents effects on the structure of Zn(II) complexes and 1D 4,4’-bipyridine derived coordination polymers, Inorganica Chimica Acta, 2020, 500, https://doi.org/10.1016/j.ica.2019.119218

