Stable metallacarboranes as potential chemotherapeutic agents
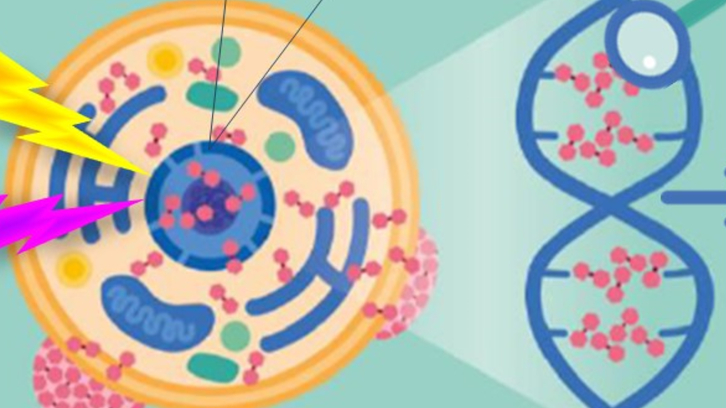
Stable metallacarboranes can become an alternative to current cancer treatments and with fewer side effects, but for them to be effective, they need to accumulate in the nuclei of cancer cells. A team from the ICMAB has investigated how one of these compounds interacts with DNA and proposes that it may be an attractive candidate for the development of new anticancer drugs.
Current cancer treatments often use drugs that interfere with DNA replication, by intercalating between base pairs, binding to the DNA groove, or forming covalent bonds with nitrogenous bases. Although these strategies are effective, they can have significant side effects. For this reason, it is crucial to develop new compounds with alternative and more specific mechanisms of action.
In this context, stable metallacarboranes have emerged as a promising alternative. In addition to their potential as chemotherapeutic agents, they can be used in innovative therapies such as boron neutron capture therapy (BNCT), which uses the boron-10 isotope, or proton-boron fusion therapy, which uses 11B and proton beams. Both strategies require boron compounds to preferentially accumulate in the nuclei of cancer cells to maximize their effectiveness.
Biomaterial preparation process
This study investigates a new form of interaction between boron-rich compounds and double-stranded DNA (ds-DNA), focusing on a specific molecule: [3,3′-Fe(1,2-C₂B₉H₁₁)₂]⁻, known as [o-FESAN]⁻. Unlike classic intercalators—typically flat, neutral, or positively charged molecules—[o-FESAN]⁻ is a compact, three-dimensional, anionic, and amphiphilic molecule. Despite having a negative charge, it manages to interact with ds-DNA, which is also negatively charged, through unusual anti-electrostatic interactions (such as C–H···O–P hydrogen bonds), overcoming the expected electrostatic repulsion from the phosphate groups on the outer ds-DNA backbone.
Furthermore, when multiple [o-FESAN]⁻ molecules approach ds-DNA, they form self-assembled structures that intercalate into the double helix, mainly in guanine-rich regions. This assembly includes additional interactions such as C–H···O–C, N–H···H–B, and C–H···H–B bonds, which stabilize the compound’s binding to ds-DNA. The result is the formation of aggregates with four [o-FESAN]⁻ molecules per base pair.
These findings indicate that [o-FESAN]⁻ can not only penetrate the cell nucleus without the need for transporters, but also binds efficiently to ds-DNA. This makes it an attractive candidate for the development of new multimodal anticancer drugs, with the potential to enhance treatments such as radiotherapy, and offer advantages in terms of specificity and efficacy. The study confirms these results through various experimental techniques.
References
Clara Viñas i Teixidor. Boro: de sus orígenes en el desierto a aplicaciones pioneras en energía, materiales y medicina, An. Quím. RSEQ, 2025, 121 (1), 19-24. http://doi.org/10.62534/rseq.aq.2008

