Recyclable and eco-compatible silica supported chiral organocatalysts
Catalysts are substances that increase the rate of chemical reactions. They are of crucial importance in the chemical industry, because in its absence many chemical processes do not occur or the use of very high temperatures and/or very long reaction times would be required. Moreover, one of the 12 principles of green chemistry refers to catalysis. The catalytic processes contribute to reduce waste and to develop a cleaner chemistry.The chiral organocatalysts are purely organic substances (they do not contain metal complexes) capable of selectively promote chemical reactions with asymmetric induction in the final product.
This property of asymmetry is important in many applications, especially in the synthesis of pharmaceuticals, where the product requires a proper configuration, as its enantiomer (mirror image) can be inactive or even show adverse effects on health. In pharmaceutical chemistry, organocatalysis offers the advantage of avoiding the presence of toxic trace metal species in the synthesized drugs.
Although the catalyst is added in a much smaller amount than reagents, it is not consumed in the reaction and it appears unchanged at the end of the process, often its separation from the final product is cumbersome (chromatographic separation). Moreover, its recovery and reuse is desirable from the economic and environmental points of view. The immobilization of the catalyst on an insoluble support allow its separation by a simple filtration in order to be re-used again, while simplifying the purification of the reaction product.
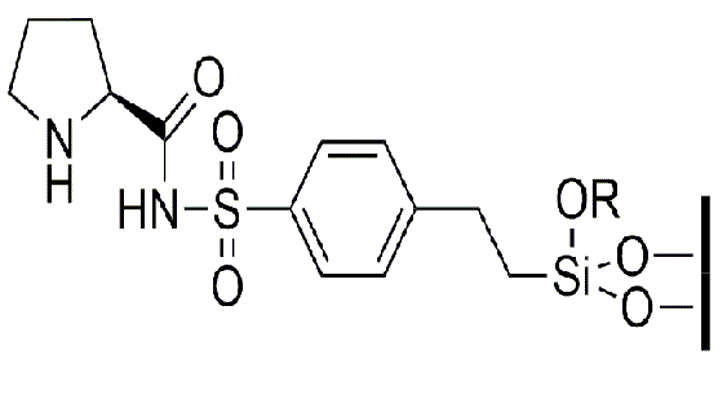
We have prepared several silica-supported organocatalysts from the natural amino acid L-proline as a chiral precursor. First the precursor has been derivatized to obtain two compounds bearing triethoxysilyl groups, from which several hybrid silicas were prepared by applying the sol-gel method to these silylated monomers or by grafting to previously synthesized mesostructured silica. These materials have been used as efficient, selective and recyclable catalysts in asymmetric aldol reactions.
These are chemical processes in which two carbonyl compounds react to form a new carbon-carbon bond and produce a new molecule. The reactions have been carried out in water at room temperature and using a small amount of organocatalysts (10-20 mol%), fulfilling the requirements of green chemistry. It has been observed that the nature and length of the linker between the silica matrix and the catalytic fragment, and the derivatisation site on the organic moiety, have an influence on the activity and selectivity of the silica-supported. organocatalysts.
Universitat Autònoma de Barcelona
References
M. Ferré, X. Cattoën, M. Wong Chi Man, R. Pleixats, Recyclable silica-supported proline sulphonamide organocatalysts for asymmetric direct aldol reaction, ChemistrySelect, 6741-6748, 2016, 1, DOI: 10.1002/slct.201601859

