Novel antibiotic discovery for bacterial biofilm eradication trough the generation of a peptide library
The lack of awareness and the inappropriate use of antibiotics have promoted bacterial resistance. Therefore, the development of novel antimicrobial agents is a major priority worldwide. Luckily, our own immune system displays a wide repertoire of antimicrobial proteins and peptides that present a fast and mutifaceted antimicrobial mechanism of action; being a useful source to develop new peptide-based alternative to conventional antibiotics.
Dr. Ester Boix laboratory focusses on the functional and structural characterization of the human ribonucleases, belonging to a family of proteins with key roles in the immune system of vertebrates. Specifically, ribonuclease 3, also known as eosinophil cationic protein (ECP), is a highly cationic protein secreted by eosinophils during infection. ECP displays a high affinity towards lipopolysaccharides, anionic molecules present at the cell wall from Gram-negative bacteria. ECP interaction at the bacterial surface triggers a self-agglutination process that promotes cell lysis and death. Strikingly, the N-terminal region of the protein is able to reproduce the antimicrobial properties of the whole parental protein.
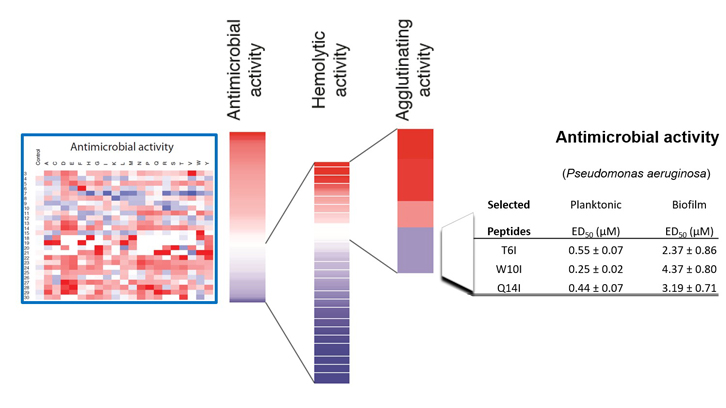
In this work we present a 500 peptide library that was generated through the SPOT technology developed in Dr. Rudolf Volkmer’s laboratory at the Freie Universität of Berlin. This technology facilitates the synthesis of a large array of peptides attached to a support membrane allowing the simultaneous characterization of a large number of peptides. The library analysis suggests that ECP N-terminus has already been selected by evolutionary pressure to provide antimicrobial activity with no toxicity towards the host cells. On the other hand, the positional scanning analysis revealed the crucial residues that could be replaced inducing a drastic increment of the peptide antimicrobial activity and decreasing the cytotoxicity towards the host cells. A combination of the agglutinating and lytic activities of the peptides provided novel and potent improved peptides able to fully eradicate bacterial biofilms. The efficacy of this peptide was further evaluated against Pseudomonas aeruginosa, being the discovery of novel drugs against this species a top priority for the World Health Organization.
The peptide has been patented and it’s efficacy against mouse infection model will be tested soon. (Project funded by Programa Indústria del Coneixement, AGAUR: 2016 PROD-00060, co-financed by FEDER funds).
This work was possible thanks to the international collaboration with Dr. Rudolf Volkmer (Freie Universität Berlin) and Dr. David Andreu (Universitat Pompeu Fabra).
Faculty of Biosciences
Universidad Autónoma de Barcelona
References
Pulido D, Prats-Ejarque G, Villalba C, Albacar M, Moussaoui M, Andreu D, Volkmer R, Torrent M, Boix E. Positional scanning library applied to the human eosinophil cationic protein/RNase3 N-terminus reveals novel and potent anti-biofilm peptides. Eur J Med Chem. 2018 May 25;152:590-599. doi: 10.1016/j.ejmech.2018.05.012. Epub 2018 May 8. PubMed PMID: 29763807.

