New route to synthesize benzothiazines
A large number of common chemical products such as medicinal drugs are based on heterocyclic organic compounds. In this work we describe a simple method for the preparation of benzothiazines, heterocycles having a bicyclic core. The method uses the commercial 2-iodoaniline as the starting material. This methodology stands out in its versatility and high product yields. The next step will consist in studying the biological activity of the newly obtained products.
Heterocylcic compounds are organic molecules with a remarkable repercussion in our society. Most of the currently used drugs contain at least one heterocycle in their structure, a fact which demonstrates their contribution to the biological activity. Agriculture and dye industry are other examples of areas where heterocycles play an important role.
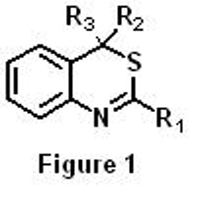
4H-3,1-benzothiazines are compounds with a bicyclic system composed of a benzene ring fused to another ring with two heteroatoms, a sulfur and a nitrogen (Figure 1). It has been found that these compounds have several applications in some of the aforementioned fields and therefore, it is of great interest to find new efficient and simple synthetic routes to obtain them.
Our work describes a direct and versatile synthesis of benzothiazines starting from the commercially available 2-iodoaniline (Scheme 1). The first step consists of introducing the R1 chain in the form of an amide through a classical condensation reaction that proceeds with high yields. The following Mizoroki-Heck reaction with ethyl acrylate or acrylonitrile introduces the Michael acceptor-type moiety essential to perform the final cyclisation. It is interesting to mention that while reactions using ethyl acrylate give only the product with E stereochemistry, reactions performed with acrylonitrile give both E and Z diastereomers. Finally, the conversion of the amide group to the corresponding thioamide using the Lawesson Reagent (LR) induces an intramolecular S-conjugate addition that affords the desired 4H-3,1-benzothiazines. This last step requires different amounts of LR as well as different reaction times depending on the electronic and steric properties of the substituent R1.
Scheme 1. The present work describes a direct and versatile synthesis of benzothiazines starting from the commercially available 2-iodoaniline.
This synthesis presents several advantages compared to the previously described routes such as its simplicity, high yields or versatility, giving that R1 could be any alkyl or aryl chain and Z any electron-withdrawing group like carbonyl, nitrile or phosphonate. Therefore, the properties of the material can be easily tuned by varying the substituents. Thus, this method allows us to synthesize a broad range of benzothiazines, promising candidates to be biological active compounds. Will any of these new molecules be a future medicament, insecticide or monomer of a new interesting material? This question has to be answered by testing them. Now is the turn of experts in other research areas.
References
A Straightforward Synthesis of Benzothiazines. Gimbert, C., Vallribera, A. Org. Lett., 2009, 11 (2), 269-271 DOI: 10.1021/ol802346

