New recyclable and eco-compatible organic catalysts
Chemistry. Carbon-carbon bond forming reactions under mild conditions constitute a powerful synthetic tool, especially when asymmetry is induced in the final product without the use of metal complexes. In this work we present a recyclable hybrid silica material derived from a chiral prolinamide and its application as efficient organocatalyst in asymmetric aldol and Michael reactions.
Asymmetry can be induced in a chemical transformation by using organic molecules as chiral organocatalysts in carbon-carbon bond forming reactions. This property may be important and critical for medical applications, where the use of metallic species is currently being reduced to avoid undesired toxic metal traces.
Although a catalyst is not consumed during the process, its separation from the final products is not always easy. Therefore the recovery and reuse of this type of molecules remains a scientific challenge for economical and environmental relevance. In this context, one of the most widely applied strategies consists in the immobilization of the organocatalyst onto a polymeric support, which allows a simple separation by filtration and an easy purification of the final products.
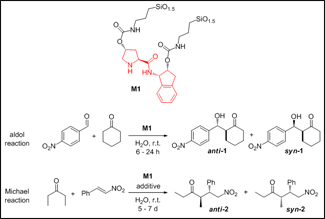
In our research we have chosen silicon dioxide as inorganic support because of its high thermal, chemical and mechanical stability. The catalytic function of the new material arises from the organic part, which is a prolinamide molecule (in red in Figure) permanently anchored to the inorganic network. The new material M1 has been prepared by a sol-gel hydrolysis and polycondensation from a silylated precursor, it has been characterized with several techniques and it has been applied in asymmetric aldol and Michael reactions (Figure).
With a very simple experimental procedure M1 gave excellent yields, good anti/syn ratios and remarkable enantiomeric excesses. The results obtained were similar to those reported for non-immobilized prolinamides, but with the advantage of recycling up to five consecutive runs without significant loss in activity or selectivity. Moreover, material M1 fits the green chemistry requirements since reactions are performed in water, at room temperature and with low organocatalyst loadings (2-16 mol%).
References
“Prolinamide bridged silsesquioxane as an efficient, eco-compatible and recyclable chiral organocatalyst” Monge-Marcet, A.; Pleixats, R.; Cattoën, X.; Wong Chi Man, M.; Alonso, D. A.; Nájera, C. New J. Chem. 2011, 35, 2766-2772.

