Ligands that contain fluorine
Fluorinated ligands play an important role in bilogical activity [i, ii]. Especially those containing trifluoromethyl groups, play an important role in medicines and agrochemicals [iii, iv].
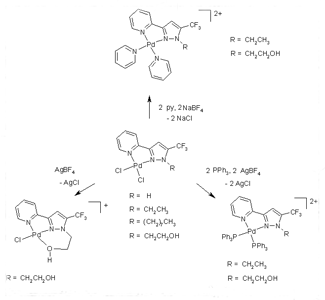
In recent years, we have developed general synthesis of 1,3,5-substituted pyrazole derived ligands, and focussed the research on the development of methods for regioselective synthesis [v]. In particular, we have synthesized N-alkyl-3-pyridine-5-trifluoromethylpyrazole derived ligands substituted with different groups in N1 position. With these ligands we report the reaction front Pd(II). The reaction of the ligands with [PdCl2(CH3CN)2] gives complexes [PdCl2(L)] (Figure 1). The stoichiometries of all complexes are independent of the M:L molar ratio. These complexes were characterized by elemental analyses, spectroscopic techniques, mass spectrometry, and X-ray diffraction methods. The metal atom of each structure is surrounded by an identical core composed by one L coordinated via one pyrazole nitrogen and one pyridine nitrogen, finishing the coordination of the metal with two chlorine ligands in cis disposition (Figure 2). The spectroscopic techniques are IR, 1H NMR, 13C{1H} NMR and 19F{1H} NMR. The 19F{1H} NMR spectra show a signal between -60.2 and -61-7 ppm, for the CF3 group, comparable to those found for other complexes described in the literature [vi].
Treatment of [PdCl2(L)] with pyridine (py) and NaBF4 or triphenylphosphine (PPh3) and AgBF4 yielded [Pd(L)(py)2](BF4)2 and [Pd(L)(PPh3)2](BF4)2, respectively (Figure 1). The 1H NMR and 13C{1H} NMR spectra of both complexes show that the two monodentate ligands coordinated to Pd (py, PPh3) are non-equivalent, and two signals can be observed.
The 31P{1H} NMR spectra for [Pd(L)(PPh3)2](BF4)2 complexes consist of broad doublet signals with chemical shifts in the usual range for Pd(II) complexes (36.1-33.9 ppm), indicating that both PPh3 groups are non-equivalent.
Finally, the reaction of the complex [PdCl2(L4)] (L4 = 2-(3-pyridin-2-yl-5-trifluoromethylpyrazol-1-yl)ethanol) with 1 mol of AgBF4 in CH2Cl2 yields [PdCl2(L4)](BF4). In this complex the ligand acts as tridentate, coordinated to Pd(II) by Npyrazole, Namino and Oalcohol finishing the coordination of the metal with one chlorine ligand.
i Y. Kabayashi, I. Kumadaki, Acc. Chem. Res. 11 (1978) 197.
ii R. Filler, Y. Kabayashi, Biomedical Aspects of Fluorine Chemistry, Kodansha Ltd, Tokyo, Japan, 1982, p. 246.
iii C. Heidelberger, N.K. Chaudhuri, P. Danneberg, D. Mooren, L. Giesbachl, R. Duschinsky, R.J. Schnitzer, E. Pleven, J. Scheiner, Nature 179 (1957) 663.
iv A.V. Fokin, A.F. Kolomiyets, J. Fluorine Chem. 40 (1988) 247.
v V. Montoya, J. Pons, V. Branchadell, J. Ros, Tetrahedron 61 (2005) 12377.
vi S.P. Singh, D. Kumar, B.G. Jones, M.D. Threadgill, J. Fluorine Chem. 94 (1999) 199.
Vanessa Montoyaa, Josefina Ponsa, Jordi García-Antóna, Xavier Solansb, Mercè Font-Bardíab, Josep Rosa
a) Departament de Química, Facultat de Ciències, Unitat de Química Inorgànica, Universitat Autònoma de Barcelona, 08193-Barcelona, España
b) Cristal·lografia, Mineralogia i Dipòsits Minerals, Universitat de Barcelona, Martí i Franquès s/n, 08028-Barcelona, España
References
Montoya, Vanessa; Pons, Josefina; Garcia-Anton, Jordi; Solans, Xavier; Font-Bardia, Merce; Ros, Josep. Pd(II) complexes containing N-alkyl-3-pyridine-5-trifluoromethyl pyrazole ligands: Synthesis, NMR studies and X-ray crystal structures. INORGANICA CHIMICA ACTA, 360 (2): 625-637 FEB 1 2007

