Four zinc(II) and cadmium(II) compounds with the benzoic acid
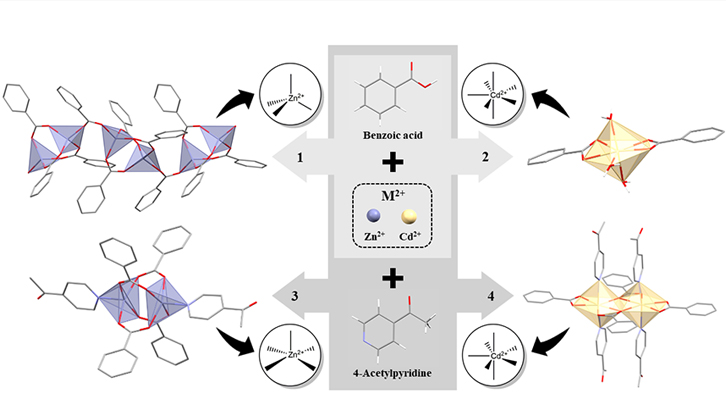
The present work, framed in the coordination chemistry, exposes the obtaining of 4 compounds of zinc (II) and cadmium (II). They have been extracted from two reactions and thanks to the benzoic acid that coordinates each acting like carboxylated ligand. The different geometries and coordination numbers of the resulting materials, depending on the particularities of the two metals, form very varied structures and have a wide range of applications and photochemical properties.
During the last decades, two new class of metal-organic materials (MOMs), the supramolecular coordination compounds (SCCs) and the coordination polymers (CPs), have emerged in the field of coordination chemistry. They have attracted a large interest not only for their structural versatility but also for their hierarchical assembly and their applications in many fields including catalysis, chemical separation or sensing.
The arrangement of these compounds is mainly based on the geometry of the metal center and the nature of the ligands and the intermolecular interactions. Remarkable is the effect of additional factors as metal:ligand ratio, counterions or solvent occluded molecules.
Carboxylic acids have been widely used in coordination chemistry as multifunctional ligands due to their large variety of coordination modes, yielding mono-, di, tri, polynuclear and polymeric coordination complexes. The benzoic acid has attracted notable interest because their synthesis is carried out in water as solvent, the greenest and most abundant of all solvents.
The metals with d10 electronic configuration (zinc, cadmium and mercury) confer a wide range of coordination numbers and geometries. In addition, Zn(II) carboxylate complexes are relevant in biological systems and Cd(II) carboxylates are interesting for their photochemical and photocatalytic properties.
This paper shows the reaction of MO (MO = metal oxide; M = Zn(II), Cd(II)) with benzoic acid (HBz) in water/methanol as solvent and in reflux, yields [Zn(Bz)2]n and [Cd(Bz)2(H2O)3]. In addition, the reaction between M(MeCO2)2 (M = Zn(II), Cd(II)) with HBz and an amine (4-acetylpyridine, 4-Acpy) in a 1:2:4 ratio and in methanol solvent, leads the formation of [Zn(µ-Bz)2(4-Acpy)]2 and [Cd((µ-Bz)2(Bz)2(4-Acpy)2]2.
The four compounds were fully characterized by analytical and spectroscopic techniques. Besides, their crystal structures were elucidated revealing a 1D coordination polymer, a monomer, a paddle-wheel and a dimer. These compounds present different coordination numbers and geometries: coordination number 4 and tetrahedral geometry (Zn(II)), and coordination number 7 and pentagonal-bipyramidal geometry (Cd(II)). In these compounds, the benzoate ligand exhibits different coordination modes (bidentate bridged, chelate, and both bridged and chelate). Besides, their extended structures were analyzed obtaining layers 2D and 3D net. Finally, the UV-Vis and fluorescence spectra have been recorded as well and their quantum yields calculated.
Universitat Autònoma de Barcelona.
Chemistry Department.
Inorganic Chemistry Area.
References
Laura Moreno-Gómez, Francisco Sánchez-Férez, Teresa Calvet, Mercè Font-Bardía, Josefina Pons. Zn(II) and Cd(II) monomer, dimer and polymer compounds coordinated by benzoic acid and 4-acetylpyridine: Synthesis and crystal structures Inorganica Chimica Acta, 2020, 506, https://doi.org/10.1016/j.ica.2020.119561

