A new protein-regulating system described
Carboxypeptidases are proteolytic enzymes, i.e. proteins whose function is the degradation of other proteins and peptides which actively participate in digestive processes, blood clotting and hormone growth. Normally these enzymes function outside the cells in which they are synthesised, although lately researchers have discovered subfamilies of this enzyme carrying out important cell growth regulation and control functions in the cell's interior, such as regulating different forms of tubulin, one of the main components of the cellular skeleton.
The control of enzyme activity and in particular of carboxypeptidase activity in this case is key for the correct functioning of living organisms. Curiously, organisms that are so distant in evolution such as potatoes, leeches, ticks, or intestinal worms have designed systems that regulate the function of carboxypeptidases, inhibiting their proteolytic activity and permitting feeding and growth. These regulation systems consist of other proteins which regulate enzyme activity and are known as protein inhibitors.
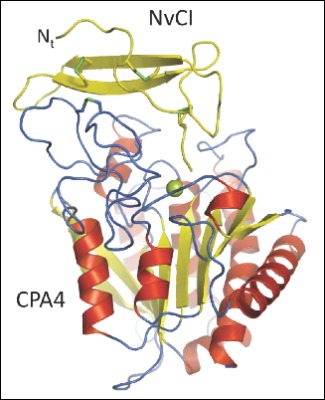
The UAB research group led by Francesc X. Aviles has spent years studying carboxypeptidases, and with the collaboration of the research group directed by David Reverter, head of a working group on protein crystallography, recently published in the prestigious Journal of Biological Chemistry a paper characterising the tridimensional structure of the complex between a human carboxypeptidase and a new protein inhibitor of a tropical marine mollusc named Nerita versicolor. This project, in which the first signer is Giovanni Covaleda, included the collaboration of a group of researchers from the University of La Havana which provided the marine organism extracts.
Only four types of carboxypeptidase inhibitors are known, all from evolutionarily distant organisms but with a common inhibitor mechanism. Of all four, the new inhibitor from Nerita versicolor is the strongest discovered until now and its tridimensional structure reveals its inhibition mechanisms with great detail. The atomic analysis of these interactions between the enzyme and the inhibitor, carried out by the protein crystallography, is very useful when designing small compounds which can interfere in the enzyme activity and which can be of great interest to the pharmaceutical and biotechnological industry.
For this study, researchers used synchrotron light, in this case in Grenoble, and they hope to soon be able to use the ALBA synchrotron light facility.
References
Crystal structure of a novel metallo-carboxypeptidase inhibitor from the marine mollusk Nerita versicolor in complex with human carboxypeptidase A4. G. Covaleda, M. Alonso del Rivero, M. A. Chávez, F. X. Avilés, D. Reverter. The Journal of Biological Chemistry, Vol. 287, Issue 12, 9250-9258, MARCH 16, 2012.


