Carbon-Gold bonds for robust molecule-electrode junctions
In the field of information technology, the increase of stored data has prompted industry and scientists to manufacture smaller electronic devices and to increase their capacity. In this direction, researchers working in the field of Molecular Electronics intend to use molecular systems as active units in these devices instead of silicon or other inorganic materials, and thus, obtain nanometric components, reaching the limits of miniaturization.
To study the behavior of these molecules, it is necessary to connect them to some conducting electrodes to measure the transport of charges through them. Although molecular electronics have progressed dramatically in the past decades, the stability, knowledge and control of the geometry of the molecular-electrode linkage at the atomic scale is a very critical and determining factor that can limit its progress, and is still under research.
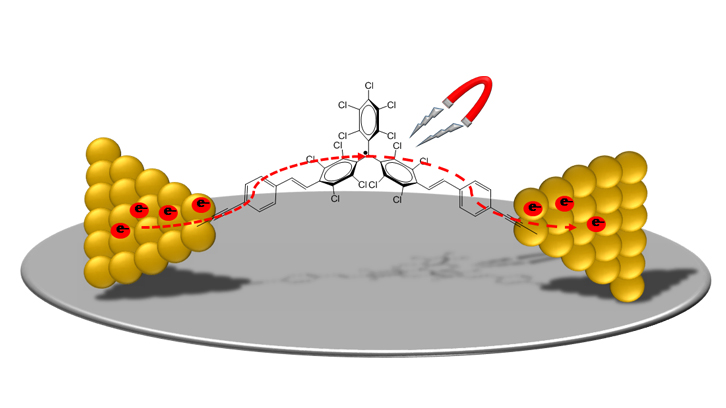
In our work we have studied the behavior of a novel stable organic radical, which is magnetic and electro-active, as a molecular cable. The studied molecule has two terminal alkyne groups that promote the linkage to gold electrodes through the formation of a covalent carbon-gold bond. This type of bond, despite being little exploited in this field, is very promising in terms of stability of the molecular-electrode interface and the molecular-electrode coupling.
First, through the preparation of self-assembled monolayers based on the novel radical, the formation of the covalent carbon-gold bond, as well as the magnetic character of the monolayer, was confirmed.
Further, we reduced the scale to study the linkage and conductance of an individual molecule, as shown in the figure. To do this, a technique known as "mechanically controlled break junction" was used. This technique allows to obtain a nanometric separation between two gold electrodes, where the molecule of interest is then chemically attached between them. This is called a molecular junction. The reproducibility of the electronic transport measurements obtained gives an idea of the formation of a stable metal-molecule bond and geometrically better defined than when other functional groups of anchorage more widely studied such as thiols, amines, pyridines or isocyanates are used. In addition, computational studies support the experimental findings.
Finally, transport measurements performed at low temperatures, below 10 kelvin (- 263 ºC) led to the observation of a phenomenon called Kondo effect, which is directly related to the presence of magnetic impurities (in this case the radical) and that appears when the electronic hybridization of the impurities and the electrons is strong.
In summary, the higher stability obtained employing carbon-gold bonds to form single molecule junctions and the detection of the magnetic character of the molecule in the junction, open the door to prepare novel and more reproducible electronic devices to potentially be applied in the field of Molecular (spin)Electronics.
References
Francesc Bejarano, Ignacio Jose Olavarria-Contreras, Andrea Droghetti, Ivan Rungger∥, Alexander Rudnev⊥, Diego Gutiérrez, Marta Mas-Torrent, Jaume Veciana, Herre S. J. van der Zant, Concepció Rovira, Enrique Burzurı́, and Núria Crivillers. Robust Organic Radical Molecular Junctions Using Acetylene Terminated Groups for C–Au Bond Formation. J. Am. Chem. Soc., 2018, 140 (5), pp 1691–1696 DOI: 10.1021/jacs.7b10019


