New properties of a human defense system enzyme identified
Ribonuclease is an enzyme secreted by leukocytes when an infection occurs, either by viruses or bacteria. In a recent study, the research group led by Ester Boix from the Department of Biochemistry and Molecular Biology identified new immunomodulatory properties of this molecule, in addition to its already known catalytic and antimicrobial activities. The mechanism of action of this multifaceted behavior, used differently depending on the type of infection, opens the doors to the design of new therapeutic agents.
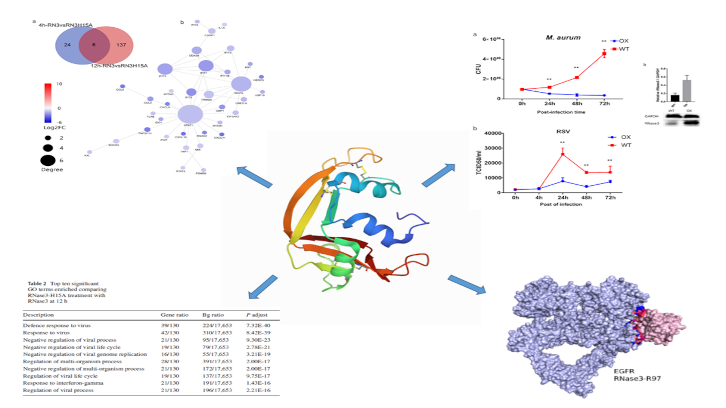
In this study carried out by the UAB research group leaded by Dr. Ester Boix we have identified novel immunomodulatory properties associated to a small human secretory protein with ribonuclease activity that participates in the innate immune response. We have observed that human RNase 3 is secreted by macrophages during infection processes. By means of a transcriptome comparative analysis of macrophages exposed to the native protein and a catalytic defective variant, novel signalling actions were identified, both dependent and independent of the protein enzymatic activity.
An initial pro-inflammatory response, unrelated to the protein catalytic activity is followed by a late response associated to the ribonucleolytic activity. Interestingly, this multifaceted behaviour is selectively applied according to the infection type. Thus, the research work demonstrates how RNase 3 overexpression protects the macrophages against both bacterial and viral infections, but using a differentiated immune response profile. During a first pro-inflammatory response, RNase 3 works as a signalling molecule that activates the epidermal growth factor receptor (EGFR).
The research work demonstrates the direct interaction of the protein with the receptor by use of a specific antibody against the receptor extracellular domain. By modelling analysis the putative key region involved in the protein-receptor binding was identified. Complementarily, the comparative analysis of the expression profile of the native and the non-catalytic variant highlighted the contribution of the enzymatic activity in immune response paths associated to antiviral action, such as the interferon-induced pathway. Next, the researchers have explored the protein role in a macrophage derived cell line infected with either mycobacteria or a single stranded RNA virus (the human respiratory syncytial virus, RSV). To note, blockage of the EGFR receptor inhibited the bactericidal but not the antiviral action of the protein.
The results emphasize the multifaceted role of this small secretory RNase from our innate immune system and provide novel tools for the structure-based design of alternative anti-infective agents. The research has been published in the Cellular and Molecular Life Sciences journal.
Lu Lu1, RanLei Wei2, Guillem Prats-Ejarque, Maria Goetz1, Gang Wang2, Marc Torrent1 & Ester Boix1
1Department of Biochemistry and Molecular Biology, Faculty of Biosciences, Universitat Autònoma de Barcelona.
2Center of Precision Medicine and Precision Medicine Key Laboratory of Sichuan Province, West China Hospital.
References
Lu, L., Wei, R., Prats-Ejarque, G. et al. Human RNase3 immune modulation by catalytic-dependent and independent modes in a macrophage-cell line infection model. Cell. Mol. Life Sci. (2020). https://doi.org/10.1007/s00018-020-03695-5


