First photochemical method to prepare polybenzoxazines in solution at room temperature
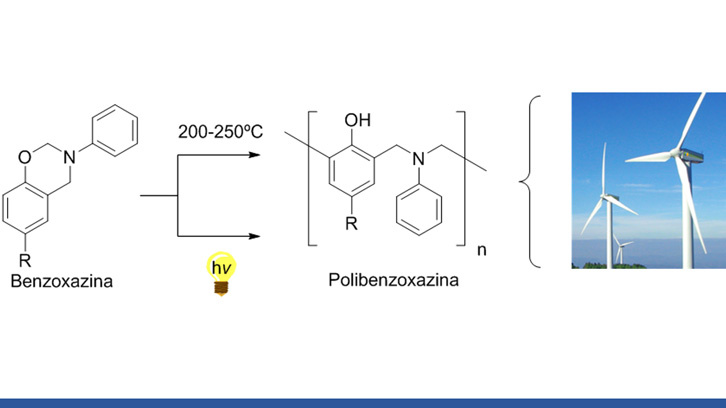
Polibenzoxazines are polymers usually prepared by heating benzoxazine monomers at high temperatures. However, this process represents a high energetic cost. That’s why the UAB Chemistry Department have created a new method of preparation based on the irradiation of benzoxazine monomers with light in an aqueous solution at room temperature. With this method similar results are obtained when comparing to the thermic method even though there are still some challenges to overcome.
Polybenzoxazines are polymers that show very interesting properties: no shrinkage during their preparation, low water absorption, low surface energy, and very resistant to chemicals and fire. Generally they are prepared by heating benzoxazine monomers at high temperatures (> 200 oC), what represents a high energetic cost for industries.
Previous studies of our group make us think that the polymerization could be initiated by irradiation of monomers with light in mixtures of organic solvents with water; conditions in which the O-C bond could be broken (Scheme 1).
We focused in three aims. First of all we chose and prepared best fitting monomers regarding their light absorption. These monomers would be the future polymer building blocks. Then, in a second step, we designed the photochemical system optimizing different parameters like light source, concentration, reaction media... We probed that the presence of water in the reaction media was required, being possible to performed the reaction only in this media. Benzoxazines containing different functional groups in the phenolic ring (R) could be polymerized in high conversions. Only benzoxazines that reduced the electron density of the ring did not work.
It is remarkable that this method was carried out at room temperature, without the addition of any metallic catalyst, acid or base, making even more sustainable the whole process (Figure 1).
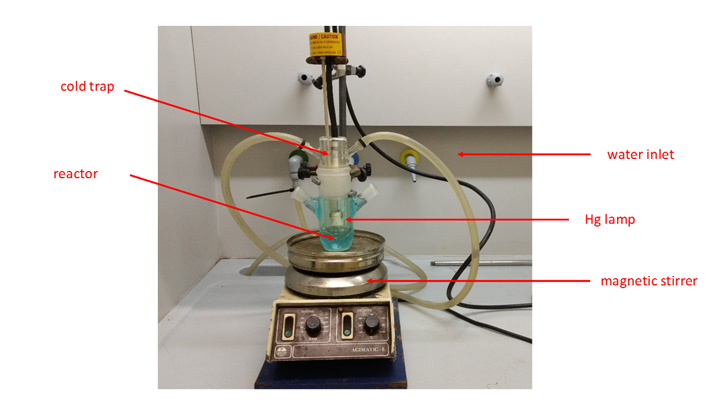
Figure 1. Reaction assembly.
In the final stage photochemically obtained polymers were compared to thermal ones, being observed that the first ones were composed by chains of similar lengths, low crosslinked among them. This could allow a great control over the prepared material, since low or null levels of crosslinking are characteristic of thermoplastic materials, which could be melted and injected into a mold. Once the desired shape was obtained, the material could easily be heated, increasing its crosslinking, rising up the average molecular weight and making it more resistant.
The research is pioneer, but there are still some challenges to overcome, such as scaling up the procedure at higher concentrations or studying in depth the properties of the obtained polymers.
Jordi Salabert, Rosa María Sebastián i Jordi Marquet
Department of Chemistry
Universitat Autònoma de Barcelona
Universitat Autònoma de Barcelona
References
1Ishida, H.; Agag, T. (2011). Handbook of Benzoxazine Resins; Elsevier: Amsterdam.
2Cayón, E.; Marquet, J.; Lluch, J. M.; Martin, X. (1991). Use of intramolecular coulombic interactions to achieve impossible reactions. Photochemical cleavage of 4-nitrophenyl ethers. J. Am. Chem. Soc, 113, 8970-8972.
3Salabert, J.; Sebastián, R. M.; Marquet, J. (2018). Photochemical polymerization of N-phenyl mono 1,3-benzoxazines in aqueous media. Macromolecules, 51, 3672-3679. DOI: 10.1021/acs.macromol.8b00171.


