Targeted resequencing reveals rare variants enrichment in Multiple Sclerosis susceptibility genes
In order to broaden the map of the genetic variants involved in Multiple Sclerosis (MS) disease and thus explore its origin, the group of the Neurology-Neuroimmunology Service from the Multiple Sclerosis Center of Catalonia (Cemcat) and from the Research Institute Vall d’Hebron (VHIR) of the University Hospital Vall d’Hebron has studied the role of the variants rarely associated with contracting MS, specifically located in 4 of the 14 genes analyzed and linked to MS. Is relevant the behavior of the rare variants located in the RGS1 gene, as they are the only ones that cause functional changes in patients carrying these variants.
Genome-wide association studies have identified approximately 200 common genetic variants associated with the risk of multiple sclerosis (MS). However, the contribution of rare variants to the genetic component of the disease is unknown. Based on this, the objective of the present study was to investigate the role of rare variants in the risk of MS.
A DNA resequencing was performed on the following fourteen genes associated with the disease: Fc receptor like 1 (FCRL1), regulator of G protein signaling 1 (RGS1), translocase of inner mitochondrial membrane domain containing 1 (TIMMDC1), hematopoietically expressed homeobox (HHEX), C-X-C motif chemokine receptor 5 (CXCR5), lymphotoxin beta receptor (LTBR), Ts translation elongation factor, mitochondrial (TSFM), galactosylceramidase (GALC), TNF receptor associated factor 3 (TRAF3), signal transducer and activator of transcription 3 (STAT3), TNF superfamily member 14 (TNFSF14), IFI30, lysosomal thiol reductase (IFI30), CD40 molecule (CD40) and cytochrome P450 family 24 subfamily A member 1 (CYP24A1). The study included a total of 545 MS patients and 550 healthy controls and five centers belonging to the Spanish Network on MS (REEM) took part on. Four regions enriched for rare variants were identified in the CYP24A1, FCRL1, RGS1 and TRAF3 genes, which were significantly associated with MS.
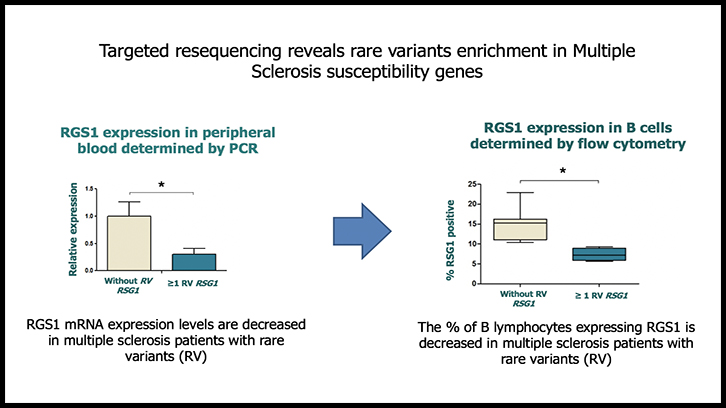
In order to study the functional consequences of the rare variants identified in these four genes, gene expression studies were first performed by real-time PCR in peripheral blood mononuclear cells (PBMC) of patients with MS with and without the rare variants. RGS1 expression was found to be significantly decreased in PBMC of patients carrying RGS1 rare variants compared to patients without rare variants. No significant differences in the expression of CYP24A1, FCRL1 and TRAF3 were observed between both groups of patients. Immunophenotyping and subsequent analysis by flow cytometry in different peripheral blood cell populations showed a significant decrease in the expression of RGS1 in B lymphocytes from MS patients carrying RGS1 rare variants.
Finally, considering that RGS1 is a gene induced by interferon-beta, in vitro studies of gene expression were also performed in the presence of interferon-beta, which showed a lower expression of RGS1 induced by interferon-beta in patients carrying rare variants. Overall, the data obtained suggests that rare variants accumulation is associated with functional consequences in MS patients such as decreased RGS1 mRNA expression levels in PBMC, reduced percentage of B lymphocytes expressing RGS1, and lack of induction in RGS1 gene expression by interferon-beta.
Manuel Comabella López 1,2
1 Medicine Department. Universitat Autònoma de Barcelona (UAB).
2 Multiple Sclerosis Center of Catalonia (Cemcat).
References
Gil-Varea, E., Spataro, N., María Villar, L., Tejeda-Velarde, A., Midaglia, L., Matesanz, F., Malhotra, S., Eixarch, H., Patsopoulos, N., Fernández, Ó., Oliver-Martos, B., Saiz, A., Llufriu, S., Ramió-Torrentà, Ll., Quintana, E., Izquierdo, G., Alcina, A., Bosch, E., Navarro, A., Montalban, X., Comabella, M. Targeted resequencing reveals rare variants enrichment in multiple sclerosis susceptibility genes. Human Mutation, Variation, Informatics and Disease. 2020 https://doi.org/10.1002/humu.24016 [Epub ahead of print] PMID: 32196808


