A new cellular mechanism strengthens intrinsic resistance to antibiotics in Pseudomonas aeruginosa
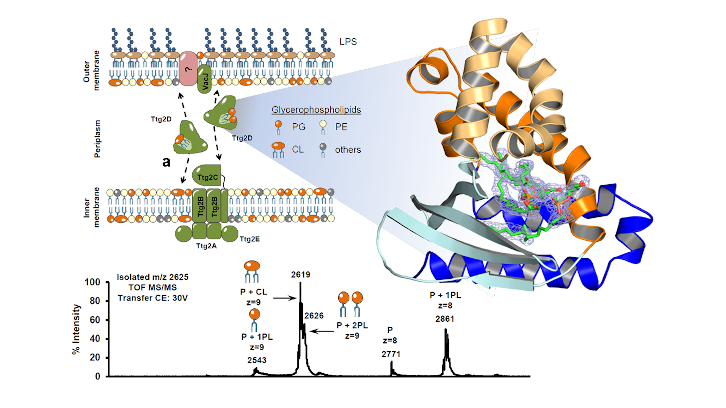
UAB researchers in collaboration with researchers at IRB Barcelona elucidate the function of the Pseudomonas aeruginosa substrate-binding protein Ttg2D in maintaining phospholipid asymmetry between the outer and inner membrane layers. In addition they have identified the role of this system in the intrinsic resistance of these bacteria to antibiotics.
Intrinsic antibiotic resistance is a naturally occurring phenomenon present in all bacterial species that challenges antibiotic chemotherapy. Pseudomonas aeruginosa, one of the most important human pathogens, exhibit intrinsic resistance to various antimicrobial agents, and multidrug-resistant (MDR) strains are a major cause of concern for public health. The basis for the inherently high resistance of these microorganisms is primarily their low outer-membrane permeability. Gram-negative bacteria have inner and outer membranes that differ in phospholipid composition.
In this context, our research group from UAB's Institut de Biotecnologia i de Biomedicina (IBB) and Department of Genetics and Microbiology and from the Institute for Research in Biomedicine (IRB Barcelona) have shown, using X-ray crystallography and mass spectrometry, that the periplasmic substrate-binding protein Ttg2D in P. aeruginosa can carry two diacyl glycerophospholipids or a cardiolipin molecule. Such simultaneous binding of two phospholipids or cardiolipin represents a novel trafficking mechanism between both membranes among Gram-negative bacteria. In addition, we could classify structurally this protein family as forming a new two-domain architecture and substrate-binding-protein structural cluster. Through the use of gene-knockout experiments in P. aeruginosa MDR clinical strains, we also identified a role for Ttg2D in resistance against antibiotics that use a lipid-mediated pathway into the cell.
The discovery of new elements of the intrinsic resistome of bacterial pathogens opens the possibility of tackling MDR in new ways, through different drug targets. In particular, the integrity of the bacterial membrane is a recognized target for novel antibacterial chemotherapies. The substrate-binding protein characterized in this study may be a candidate for an antibiotic intervention based on the specific blocking of the maintenance of membrane integrity.
Institut de Biotecnologia i de Biomedicina (IBB)
Departament of Genetics and Microbiology
Universitat Autònoma de Barcelona
References
Yero D, et al. The Pseudomonas aeruginosa substrate-binding protein Ttg2D functions as a general glycerophospholipid transporter across the periplasm. Commun Biol. 2021 Apr 9;4(1):448. doi: 10.1038/s42003-021-01968-8. PMID: 33837253.
Research authors:
Daniel Yero (a,b), Mireia Díaz-Lobo (c), Lionel Costenaro (a), Oscar Conchillo-Solé (a,b), Adrià Mayo (a), Mario Ferrer-Navarro (a), Marta Vilaseca (c), Isidre Gibert (a,b) i Xavier Daura (a,d).
(a) Institut de Biotecnologia i de Biomedicina (IBB), UAB; (b) Departament de Genètica i de Microbiologia, UAB; (c) IRB Barcelona; (d) ICREA.
Isidre.Gibert@uab.cat; Xavier.Daura@uab.cat


