New recoverable metathesis catalysts
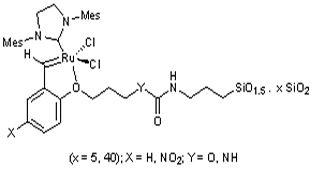
The preparation of several organic-inorganic hybrid materials by sol-gel process derived from Hoveyda-type monomers is described. One of them presents a nitro group at the para position with respect to the alkoxy moiety. These materials were treated with Grubbs catalysts to generate the corresponding Hoveyda-Grubbs carbene ruthenium complexes covalently bonded to the silica matrix, which were tested as recyclable catalysts for diene and enyne ring-closing metathesis (RCM). Electronic effects of the nitro group resulted in enhanced activity of the catalyst. Whereas the recyclability decreased in RCM of dienes, the presence of this electron-withdrawing group was highly advantageous for the RCM of enynes, the reusability being greatly improved.
Olefin and enyne metathesis have emerged in the last decade as very important reactions in the field of organic synthesis. Their enormous success can be attributed to the discovery of several well defined metal carbenes, the Grubbs catalysts, which have proven to be efficient and selective promoters. A second generation of them, specially the Hoveyda-Grubbs catalysts, has shown an even greater reactivity, as well as excellent stability and ease of recovery. Several studies have described the influence of electronic and steric effects in these complexes. Enhanced reactivity can be achieved with several variants bearing strong electron-withdrawing groups in the para position with respect to the alkoxy or alkylidene group.
Concerning the recovery, one of the most used strategies consists in the immobilization of the complex on an insoluble polymeric support. A simple filtration at the end of the reaction permits an easy separation of the product and recovery of the catalyst. Organic polymers have been the most frequently used supports, but hybrid organic-inorganic materials formed by catalytic species covalently anchored to silica have superior chemical, mechanical and thermal stability. The sol-gel hydrolytic condensation of suitable organo-alkoxysilanes is a convenient method to prepare new hybrid materials with targeted properties, such as high surface area or material structuration.
Figure 2. Metathesis reactions tested.
In this work new materials have been prepared from monosylilated monomers bearing a nitro group in para position with respect to the alkoxy moiety, and their efficiency as catalysts and their reciclability has been compared to other materials where the nitro group is missing.
At first, three new monosylilated monomers with the desired groups have been synthesized. Then several materials have been prepared (Figure 1), either through grafting to commercial silica or to MCM-41 (high porosity and surface area), or by direct synthesis with the sol-gel process. In the later case a surfactant has been added to some of the reactions as structuring agent in order to obtain a high surface area. These materials have been charged with the Grubbs ruthenium complex and have been tested in several olefin and enyne metathesis reactions (Figure 2). These catalysts are efficient promoters of the reaction, even on more challenging substrates giving rise to tri- and tetrasubstituted olefins. The conditions are milder and the reciclability better than the one found with other supported catalysts described in the literature.
In all cases, materials prepared from sol-gel are superior to those coming from grafting. The structuration achieved with the use of surfactants has also improved the activity, while the presence of the electron-withdrawing nitro group enhanced the reactivity in olefin metathesis, but reciclability was poorer. On the contrary, in enyne metathesis the nitro group has given rise to both faster reactions and better reciclability, constituting the best reusable catalyst described for this reaction.


