Study of highly efficient molecules to improve organic synthesis
Based on a combined experimental and computacional study, authors of this work analized the effectiveness of some groups of molecules derivated from pirazol, used as precatalists in organic synthesis developed by Heck reactions.
Several [PdCl2(L)] complexes, where L is a pyridylpyrazole ligand (Figure 1), have been used as precatalists in Heck reactions between phenyl halides and tert-butil acrylate. The Heck reactions is one of the most widely used palladium-catalyzed reactions in organic synthesis [i, ii, iii]. The used ligands differ from each other in the substitution at N1.
The synthesis and characterization of several pyridylpyrazole-derived ligands with different substitutions in the 3,5-positions have been reported in the literature [iv, v, vi], and the reactivity of some of these ligands with Pd(II) has been studied in our laboratory [vii, viii, ix].
A common problem in the coordination chemistry of the pyridylpyrazole ligands to metal ions is the low solubility of these complexes in organic solvents. This solubility can be increased by the incorporation of an alkyl or hydroxyethyl group at the N1 position of the pyrazole ring.
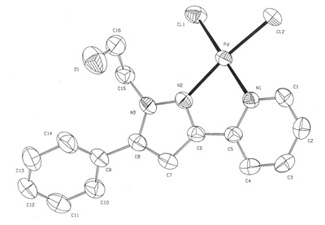
In recent papers, we have reported the synthesis and characterization of several N-alkyl-3,5-pyrazole derived ligands with ethyl, octyl and hydroxyethyl groups at the N1-position. We have also studied the reactivity towards Pd(II). The best results are obtained when this substituent is a hydroxyethyl group (Figure 2), and the corresponding complexes yield good results even for the Heck reaction using of chlorobenzene as the aromatic halide.
Theoretical studies have shown that the presence of an OH group in the N1 substituent favours Pd-X dissociation, since it stabilizes the resulting cationic complex and the dissociation becomes thermodynamically favourable even in the absence of a coordinating solvent molecule.
Figure 2. The best results are obtained when this substituent is a hydroxyethyl group.
Vanessa Montoya (a), Josefina Pons (a)*, Vicenç Branchadell (b), Jordi García-Antón (a), Xavier Solans (c), Mercè Font-Bardia (c) and Josep Ros (a)
a) Departament de Química, Unitat de Química Inorgànica, Universitat Autònoma de Barcelona, E-08193-Bellaterra, Cerdanyola, Spain
b) Departament de Química, Unitat de Química Física, Universitat Autònoma de Barcelona, E-08193-Bellaterra, Cerdanyola, Spain
c) Deparatment de Cristal·lografia, Mineralogía i Diposits Minerals, Universitat de Barcelona, Martí i Franquès s/n, 08028-Barcelona, Spain.
References
"Highly efficient pyridylpyrazole ligands for the heck reaction. A combined experimental and computational study". Montoya, Vanessa; Pons, Josefina; Branchadell, Vicenc; Garcia-Anton, Jordi; Solans, Xavier; Font-Bardia, Merce; Ros, Josep. ORGANOMETALLICS, 27 (6): 1084-1091 MAR 24 2008.


