Important advances in the study of membrane transport proteins
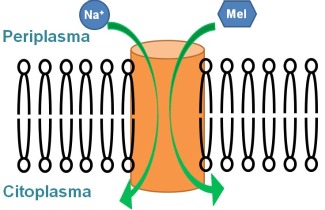
All cells need to exchange ions and small molecules with their environment. This exchange takes place in a controlled manner via membrane transport proteins located in the cell membrane. An important subset of these proteins comprises the active transport proteins, which use energy to transport the substrate against its concentration gradient. An outstanding example is the sugar transporter proteins. These proteins use the energy from ion gradients (usually sodium) or proton to transport the sugar molecules into the cytoplasm. It is, therefore, a cation-sugar co-transport.
These co-transporters facilitate the passage of the sugar and the cation from the periplasm to the cytoplasm in different stages. The transport mechanism includes a series of conformational changes that orient the protein toward the periplasmic side, allowing the binding of sodium and then the sugar, followed by a reorientation towards the cytoplasmic space, in which the sugar is first released followed by the cation. This alternation in the accessibility of the substrate-binding site is a mechanism common to many membrane transport proteins and is presumably related to changes in orientation of some of the transmembrane helices.
As a first approach to study the role of the reorientation of transmembrane helices in the function of transport proteins, bacteriorhodopsin was studied as a model membrane protein. This protein of 7 transmembrane α helices is a primary active transporter that transports protons by means of the energy of light. To determine the role played by the movements of transmembrane helices, two cysteine residues at strategic locations at the end of two adjacent helices were introduced by site-directed mutagenesis. The formation of a disulfide bond, which restricts the movement of the helices, was induced. Using different methodologies, the analysis of the function of this altered protein in comparison to the native protein, reveals that the transmembrane helices move as a rigid body (1). This conclusion can be extrapolated to other membrane proteins, as the G protein-coupled receptors (7 transmembrane α helices), or transmembrane transporter proteins. Thus, this work contributes to understanding how the transmembrane helices of such proteins move to perform their function.
To substantiate this concept in the case of transporters of small molecules, the melibiose carrier was studied. It is a protein with 12 transmembrane helices, which transports the disaccharide melibiose using a gradient of sodium. Using polarized infrared difference spectroscopy, it was shown that the orientation of transmembrane helices varies because of sodium or melibiose binding (2). This evidence reinforces the concept that some transmembrane helices of active transporters move as rigid bodies to perform their function of transport.
On the other hand, in a recent work on the melibiose transporter, published in Proc. Natl. Acad. Sci. (3), the amino acids involved in the binding of the two substrates (melibiose and sodium) were investigated. Again using infrared difference spectroscopy and also fluorescence spectroscopy, the conformational changes caused by the binding of substrates were detected. Comparing the results of selected mutants with the native protein, two aspartic acid residues involved in binding of sodium and two aspartic acid residues that interact with the molecule of sugar were identified. Using structural models of the melibiose transporter, the location of these key amino acids for substrates binding were predicted: the 4 aspartic acids are located in three different transmembrane helices. The results of this study (3), together with the polarized infrared spectroscopy work (2) indicate that the binding of substrates in the transmembrane region causes a change of the orientation of transmembrane α helices.
These studies can provide key knowledge to understand how the human homologous transporters function, which are difficult to study due to the complexity of their purification. As relevant examples are: i) the sodium-glucose transporters responsible for the reabsorption of glucose in the nephrons of the kidneys. Controlled inhibition of these transporters may be a good strategy to treat diabetes by increasing the excretion of excess glucose to the urine. ii) the glucose facilitators, responsible for the diffusion of glucose into the cells. These proteins are part of the major facilitator family, with which the melibiose transporter shares a very similar structure of 12 transmembrane helices and the ability of binding sugars.
References
1. Rosana Simón-Vázquez, Tzvetana Lazarova, Alex Perálvarez-Marín, José-Luis Bourdelande, and Esteve Padrós (2009) Cross-Linking of Transmembrane Helices Reveals a Rigid-Body Mechanism in Bacteriorhodopsin Transport. Angew. Chem. Int. Ed. 48, 1 – 4.
2. Víctor Lórenz-Fonfría, Meritxell Granell, Xavier León, Gérard Leblanc, and Esteve Padrós (2009) In-plane and out-of-plane infrared difference spectroscopy unravels helices tilt and structural changes in a membrane protein upon substrate binding. J. Amer. Chem. Soc. 131, 15094-15095.
3. Meritxell Granell, Xavier León, Gérard Leblanc, Esteve Padrós and Víctor Lórenz-Fonfría (2010) Structural insights into the activation mechanism of melibiose permease by sodium binding. Proc. Natl. Acad. Sci. USA doi:10.1073/pnas.1008649107.


