Chemistry

Photochemistry of Indigo: a long-standing controversy
Although the use of indigo as a dye dates back to at least the 7th century B.C., it was not until the late nineteenth century when its chemical structure was discovered, and 60 years more passed before spectroscopic data was obtained. The indigo molecules are not water soluble, and therefore must be reduced to a form called leucoindigo. A work has studied the characteristics of both forms of indigo to understand and explain the different photophysical and photochemical properties presented by them.
References
Moreno, M.; Ortiz-Sánchez, J.M.; Gelabert, R.; Lluch, J.M. A theoretical study of the photochemistry of indigo in its neutral and dianionic (leucoindigo) forms. Physical Chemistry Chemical Physics 15(46): 20236-20246. 2013. DOI: 10.1039/c3cp52763h.
Indigo is one of the oldest dyes used by the human race. In fact it is not even known the origin of its use though its name suggests that it was first used in India. The first written reference to indigo is found in a Babylonic stele dating from the 7th century BC. Egyptians made an extensive use of indigo as a dying agent, to paint walls or write scrolls and Caesar cites in the De Bello Gallico that Gauls painted their faces in blue in order to become immune to the enemy weapons (a use Caesar probed utterly wrong). However it was not until the end of the 19th century (1882) that the chemical structure of indigo was discovered. It took yet another 60 years (in 1942) to report the first spectroscopical data of indigo. Since then there have been a plethora of scientific studies devoted to understanding the quite unique photochemical properties of indigo that make of this molecule an unmatched efficient dying agent for all kinds of garments. In fact the most popular use of indigo well predates scientific studies, as it goes back to the gold rush era in California (circa 1873) when Levi Strauss and Jacob Davis created the blue jeans for the miners.
As seen in the accompanying figure, indigo is a neutral molecule with a quite symmetric structure. The two hydrogen atoms explicitly written in the structure are suspected of being involved in the photochemistry of indigo as they can be transferred from the nitrogen atoms they are originally bonded to, to the oxygen atoms nearby. Neutral indigo is not water-soluble so it has to be reduced to its dianionic form called leucoindigo which is also depicted in the figure. Indigo and leucoindigo have very different chemical behaviors. They absorb at quite different wavelengths (so that a solution of leucoindigo salt is not blue but reddish) and the neutral form is very stable, since after irradiation 99.7% of the indigo molecules recover their initial form without emitting light (fluorescence). Conversely, leucoindigo has a noticeable fluorescence yield of 34.8% with a large Stokes shift (difference between the wavelength of the absorbed and emitted light). In our work we have performed a thorough study using state-of-the-art theoretical chemistry tools in order to fully analyze the properties of both indigo and leucoindigo that could be relevant to fully understand their photophysical and photochemical properties.
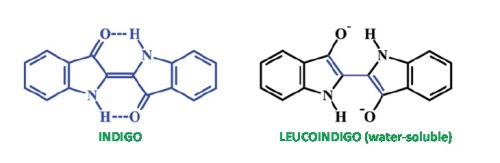
Our results indicate that for indigo the process of hydrogen transfer is not energetically favored even in the excited state accessed upon absorption of light. The fact that the molecule does not emit light after absorption means that there is a very efficient deactivation path back to the initial geometry. In theoretical terms this implies the presence of a so called Conical Intersection (CI) between the ground and excited electronic states. Previous theoretical works pointed to a CI to be found after one hydrogen had been transferred. As a novelty we have located a CI accessed by simply rotating the initial structure of indigo around the central C-C double bond. Previous calculations had discarded this possibility on the grounds that it was too high in energy, but we have shown that it may be low enough to be directly accessed following photoexcitation of the initial structure.
As for leucoindigo (which had been barely studied from the theoretical side) our results point to a far different photochemistry. Surprisingly, the most stable structure for leucoindigo is not the one shown in the figure but the one with the two hydrogen atoms bonded to the oxygen atoms. After photoexcitation to the active excited state the original structure of indigo becomes the more stable one for leucoindigo as well and two hydrogen atom transfers may take place. This explains the large Stokes shift experimentally measured and makes the photochemistry of leucoindigo much richer than the one found in the neutral species as several CIs can now play a role in the deactivation paths that follow photoexcitation.
Miquel Moreno
Department of Chemistry
2024 Universitat Autònoma de Barcelona
B.11870-2012 ISSN: 2014-6388
